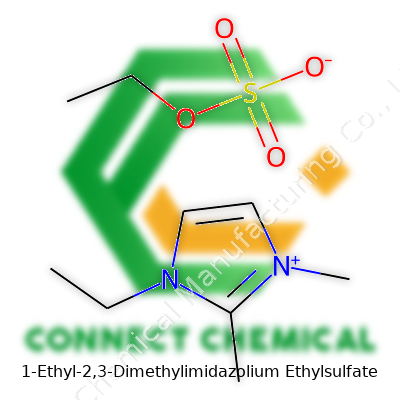
1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate: A Deep-Dive Commentary
Historical Development
Looking back, the story of 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate (EDMIS) emerges from decades of ionic liquid research. Back in the 1990s, ionic liquids mostly stayed confined to the pages of academic journals or specialty labs, regarded as exotic curiosities compared to classic solvents. As environmental regulations pressed for cleaner alternatives, chemists hunted for greener ways to replace volatile organic compounds. The imidazolium family offered a path forward thanks to their unique combination of low vapor pressure, chemical stability, and the ability to tweak properties through small structural changes. EDMIS, with its particular ethyl and methyl groups, became a practical option for labs and industry in the search for safer, more tunable solvents.
Product Overview
Sold commercially as a clear, high-boiling ionic liquid, EDMIS pops up among modern chemical suppliers in bottles ranging from a few grams to multi-kilogram drums. People handling it see its viscosity and lack of color right away. Unlike many traditional solvents, this compound stands out by avoiding the sharp odors and health concerns tied to many flammable liquids. Its growing appeal lies in its dual alkyl-imidazolium backbone and ethylsulfate anion, which set it apart from older, chloride-based salts. Research groups and specialty manufacturers favor EDMIS for its reliable performance, straightforward handling, and environmental profile.
Physical and Chemical Properties
EDMIS exists as a viscous, colorless liquid at room temperature. Melting well below 25 degrees Celsius and boiling only at much higher temperatures, it handles heat better than many organic solvents. Density sits around 1.1 to 1.2 g/cm³. Water solubility is substantial, easing clean-up and blending with aqueous systems. Many folks in the lab find that the imidazolium cation resists attack from base or acid, maintaining chemical stability even under tough reaction conditions. The low volatility makes EDMIS less likely to trigger air-quality issues or rapid evaporation, reducing the need for heavy ventilation.
Technical Specifications and Labeling
Reputable suppliers detail EDMIS with a chemical purity above 98%, supported by NMR and elemental analysis. Product labels show the CAS number, formula (C9H18N2O4S), and key safety information, including standard pictograms and GHS signal words. Instructions on storage recommend sealed bottles and ambient temperatures, away from strong oxidizers. Some bottles come with certificates tracing each batch’s properties, shining a light on impurity profiles, notably chloride or water content, which can affect certain catalytic or electrochemical experiments.
Preparation Method
Synthesis follows a robust two-stage process practiced since the turn of the century. The process starts with alkylation of 2,3-dimethylimidazole using ethyl halides to build the quaternary imidazolium core. After isolating the intermediate salt, a metathesis reaction with sodium ethylsulfate swaps out the halide for the ethylsulfate anion. This two-step route often ends with repeated washing and distillation under vacuum, leaving the product both pure and ready for demanding lab work. The simplicity of the synthetic steps is a big reason EDMIS entered commercial catalogs so rapidly.
Chemical Reactions and Modifications
Researchers often appreciate EDMIS for its robust ionic nature, which brings unique solvation properties to catalysis, organic synthesis, and even electrochemistry. EDMIS’s core stays largely inert during most standard bench reactions, leaving the imidazolium ring unscathed. Some teams try minor modifications on the alkyl side chains to tune viscosity or compatibility with specific solutes. On rare occasions, the ethylsulfate anion can undergo substitution under strong nucleophilic attack, but the main cation-anion pair proves tough under typical usage. The resilience of the core structure lets EDMIS act as both a medium and reagent in an expanding set of chemical protocols.
Synonyms and Product Names
EDMIS carries a few alternative names among suppliers, including 1-ethyl-2,3-dimethylimidazolium ethyl sulfate, [EMMIM][EtSO4], and 2,3-dimethyl-1-ethylimidazolium ethyl sulfate. Some catalogues bundle it with similar ionic liquids for ease of comparison, but the unique substitution pattern often comes highlighted to avoid confusion. The imidazolium family's naming conventions take some getting used to. People working with various methyl or ethyl combinations need to check each molecular label carefully, since subtle changes in side chains shift properties quite a bit.
Safety and Operational Standards
Users working with EDMIS often notice its lower flammability risk compared to standard solvents, but safety data sheets still call for basics: gloves, goggles, and careful handling. Local authorities in the U.S., EU, and Asia apply the GHS guidelines, classifying EDMIS as an irritant if inhaled or exposed to skin for extended periods. Long-term storage needs sealed glass or HDPE containers away from direct sunlight. I’ve seen labs struggle with the sticky residue EDMIS can leave if spilled—its high viscosity clings to benches and glassware, so early cleanup matters. Strong oxidizers or reducing agents might trigger side reactions if mixed in bulk, but most bench-scale operations run safely with good chemical hygiene.
Application Area
The true impact of EDMIS unfolds across a spectrum of modern laboratories. In green chemistry, EDMIS works as a replacement for volatile organic solvents in catalysis, extraction, and as a stable, reusable medium. It supports enzymatic reactions by stabilizing proteins, opening new doors for biocatalysis. Electrochemical researchers like its wide electrochemical window, enabling studies of electrodeposition and battery prototypes pushed to new voltage limits. In organic synthesis, EDMIS often steps up as a solvent for transition metal catalysis, C-H activation processes, or green oxidation reactions. Its miscibility with water lets process engineers design one-pot reactions or easier product separation schemes. Even material scientists jump in, applying EDMIS in polymer synthesis or nanoparticle stabilization, leveraging its ionic environment to control particle growth.
Research and Development
Across academic journals and patent filings, a rising tide of studies highlights the promise and limits of EDMIS. Universities leverage its unique solvent properties for tough syntheses; industrial labs chase energy-efficient separations. Several R&D groups push for custom-tailored derivatives, tweaking side chains to further shrink toxicity or boost specific solubilities. Others look at full life-cycle analyses, wanting to understand EDMIS’s environmental footprint from synthesis to disposal. Open databases now track hundreds of reactions using this liquid, fueling a feedback loop: each published method nudges innovators to refine protocols or swap in greener alternatives. As regulatory focus tightens on hazardous solvents, competing labs line up behind EDMIS for pilot-scale studies in pharmaceuticals, fine chemicals, and battery tech.
Toxicity Research
The broader use of EDMIS raises real questions about health and environmental impact. Early studies dove into acute and chronic exposure in aquatic and mammalian models. Several reports, including some from European REACH registration, classify EDMIS as having low to moderate aquatic toxicity. In my own conversations with lab safety staff, there’s often relief that EDMIS doesn’t volatilize into the air, though the high persistence in water means accidental spills could upset local wastewater streams if not contained. Its breakdown products, stemming mostly from slow hydrolysis of ethylsulfate, show minimal bioaccumulation in current studies, though regulatory agencies still press for more long-term results. The push for in vitro bioassays and computational toxicity models keeps climbing, as the chemical’s reach expands in commercial applications.
Future Prospects
EDMIS finds itself at the crossroads of ongoing green chemistry reform. Its low vapor pressure, chemical resilience, and expanding record of industrial testing position it as a front-runner in clean solvent design. The next wave will probably see EDMIS entering roles in CO2 capture, solid-state electrolytes, and continuous flow reactors where classic solvents struggle. Performance tweaks, new regulatory standards, and tighter toxicological controls all sit on the horizon, pushing manufacturers and university groups alike to nail down safer, faster synthetic routes. The rising tide of environmental accountability means EDMIS must clear new hurdles or adapt as new alternatives inevitably appear. Experience tells me that the willingness to integrate real-time safety data, learn from unexpected toxicology signals, and invest in waste minimization will separate this class of ionic liquids from obsolete ones. Research networks, coupled with open chemical data, will decide how EDMIS shapes—if not replaces—the next generation of industrial and academic chemistry.
Fresh Approaches to Green Chemistry
Chemistry no longer serves just giants in lab coats and massive manufacturing plants. Green solvents are popping up in smaller startups and everyday labs, and 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate stands out in this story. For many chemists, solvents have meant headaches and risk, both for their own health and for the world around them. This compound gives researchers a shot at shaking off the grip of old, hazardous solvents. Its low vapor pressure means less air pollution, less inhalation danger, and easier containment. These qualities draw both newcomers and experienced pros toward greener experiments.
Opening Doors in Cellulose Processing
Plant fibers hold promise for plastics, textiles, and even medicine. Breaking down cellulose, though, takes some muscle, and most common solvents fall short or cause environmental headaches. 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate helps solve this puzzle. Its ionic structure allows it to dissolve tough plant cellulose with far less waste and fuss than traditional acids or bases. My time in university labs showed how stubborn wood pulp can be when soaked in outdated chemicals. A clean, simple process saves time, cost, and post-reaction headaches. This isn’t just for science class. Textile factories and paper mills now hunt for ways to minimize harsh chemicals, and this compound finds its place on their shelves.
Sharpening Extraction and Separation in Industry
Big industries chase efficiency. Purifying compounds, separating valuable ingredients from complex mixtures—these challenges often create tons of chemical waste. 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate delivers in extraction and separation processes. Its ionic nature means better solubility profiles for certain organics, speeding up reaction times and boosting the amount of usable material pulled from natural feedstocks. I’ve watched extraction work get bogged down by slow-moving, toxic solvents. A more selective, less volatile option cuts down on unnecessary steps and reduces clean-up work, which matters for both budgets and job safety.
Boosting Performance in Catalysis
Catalysts give chemical reactions a jumpstart, but the real world often throws curveballs. Temperature swings, air exposure, and unwanted side effects gum up the works. 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate helps keep catalysts stable and reactive at lower temperatures, which saves both power and wear-and-tear on equipment. I remember a project that stalled out when a traditional solvent reacted with the catalyst, wrecking months of planning. Ionic liquids like this one prevent those expensive stumbles and help deliver more consistent results. For both pharmaceutical makers and specialty chemicals, that efficiency leads to better products at lower cost.
Supporting Clean Energy Innovations
The push for sustainable fuel and energy storage gives new chemistry a central role. Electrolytes for batteries, hydrogen storage, and fuel cell technology often face corrosion, low stability, or safety issues with traditional materials. 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate gives designers a stable, less flammable electrolyte with good ionic conductivity. That small change unlocks higher durability and less maintenance, which matters as green tech scales up to real-world demand. Watching these transformations helped me realize how fresh materials shape entire industries, not just single devices.
Next Steps for Responsible Chemistry
One roadblock stands out: cost. Ionic liquids can stretch a lab or company’s budget. Scale-up projects need cheaper, more available synthesis routes, and many research groups are already diving into these improvements. Regulations also come into play, as the toxicity and full environmental impact of newer chemicals still need careful evaluation. Open communication between academia, business, and regulators will keep uses for this compound both safe and far-reaching.
What You’re Working With
1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate looks like a mouthful, but it’s just one of the family of ionic liquids. Chemists like using these compounds because they don’t evaporate much, they dissolve a wide range of chemicals, and they work well in all sorts of applications from catalysis to advanced battery research. Because it doesn’t have much smell and doesn’t boil easily, it won’t fly off into the air like benzene or acetone. But that doesn’t mean it’s harmless just because you can’t smell it or see vapor rising from a beaker.
Looking at the Evidence
Based on scientific databases and published safety sheets, there isn’t a mountain of health data for this exact compound. That’s common for new specialty chemicals. What exists points to a low risk of volatility, flammability, and inhalation exposure under normal lab use. You won’t likely pass out from breathing fumes, which separates it from the old-school lab solvents. But absence of evidence isn’t evidence of absence. For ionic liquids similar to this one, skin and eye irritation appear at moderate exposure. Some reports suggest these chemicals can also be toxic to bacteria and aquatic life even at low doses. Ignoring those signs would be careless.
Why Gloves and Goggles Still Matter
Years in the lab teach a healthy respect for anything with a long name and an unfamiliar safety data sheet. It’s easy to get comfortable, especially if nothing bad happens after early contact. The truth is, skin absorbs chemicals whether you feel a burn or not. Some ionic liquids get through gloves faster than you’d guess. Standard latex might not cut it; nitrile or layered gloves will block most exposure in case the liquid spills or splashes. Even a few drops can cause skin to dry out, crack, or show a rash. Eyes need covering even more since liquids like these can sneak past safety glasses during pipetting or transfer. Once something like this lands on the sclera, you’ll feel the sting, and permanent damage can’t get fixed with a simple rinse.
Ventilation and Spills
Just because you don’t see vapors doesn’t mean you can turn off the fume hood. Accidental spills can coat benches or gloves, staying sticky for hours. These liquids sometimes travel on shoes and fingers unless staff get strict about handwashing and changing gloves. Work in fume hoods, keep surfaces clean, and manage waste with sealed containers. Once ionic liquids end up in regular waste bins, they can leach out and impact water treatment facilities, harming aquatic life. Strict waste tracking isn’t bureaucratic red tape; it’s smart stewardship for your team and the neighborhood downstream.
Better Safe Than Sorry
Training never feels glamorous, but it’s built into any lab that runs for years without injuries. Staff who know how to read a safety data sheet, recognize chemical hazard pictograms, and ask questions before uncapping a new bottle don’t wind up writing accident reports. Chemists who swap stories over coffee talk about the person who skipped one day’s protection and wound up in urgent care. Safety means planning routines, not waiting for problems. Lab managers and teachers can run drills and check gear, but it takes everyone to keep a chemical like 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate from turning into the next nasty headline.
Understanding What’s Inside the Label
Walking through the supply aisle of any lab, bottles and drums announce chemistry’s silent promise: purity and concentration. For anyone who’s ever handled materials in research, agriculture, or industry, those two numbers spell out what you can achieve with a product. Yet, too often people accept them at face value, rather than digging into why they matter so much.
PURITY AS A TRUST SIGNAL
Chemical purity refers to how much of a substance is actually what it’s supposed to be. Purity isn’t just numbers on a spec sheet. It’s the difference between consistent results and costly headaches. For instance, you’ll find that most analytical reagents reach a minimum of 99% purity. Pharmaceuticals or food-grade chemicals push for even higher numbers to limit risk. Impurities might cause downstream reactions to fail or lead to unsafe outcomes if left unchecked.
When you source materials for critical applications, slight contamination can ruin carefully developed processes. I learned the hard way in an undergraduate research project, where even a half-percent of the wrong contaminant shifted results outside tolerance. Companies that value their customers provide Certificates of Analysis. Those records show batch-by-batch purity, giving you something to hold suppliers accountable.
WHY CONCENTRATION SETS EXPECTATIONS
Concentration describes the strength—how much of the chemical is present compared to other stuff in the container. Say you buy hydrochloric acid. In the lab, a “concentrated” bottle typically means about 37% HCl by mass. Lower concentration forms work better when safer handling is important, or for specific labs that tolerate more diluted solutions.
Years ago, I ordered what I thought was a standard sodium hypochlorite for a school pool: concentration seemed right, but it ended up much lower than required. After a few swimmers complained about cloudy water, I checked and realized we required a product with at least 12% available chlorine. The seller offered only 6%. That gap didn’t just affect cleanliness; it changed the pool’s cost structure—double the product, double the work, same old result. Knowing what concentration fits your task isn’t negotiable; it forms the bedrock for operational planning.
SUPPORT FROM EVIDENCE—AND TRANSPARENCY
Product datasheets and safety labels from reputable manufacturers don’t hide concentration or purity numbers. They disclose them plainly, referencing industry standards (like ACS for analytical chemistry or USP for pharmaceuticals). These numbers are no accident. They line up with scientific consensus and regulatory guidelines. When those details aren’t easy to spot, it usually means the supplier either cuts corners or lacks proper quality systems.
Modern supply chains sometimes bring surprise impurities from upstream sources or contamination during shipment. Quality-oriented companies use third-party testing—not just in-house checks—to maintain customer trust. They update certificates regularly and field technical teams to help interpret certificates and recommend proper storage and handling.
BUILDING BETTER BUYING PRACTICES
Everyone in the chain—manufacturer, distributor, buyer—carries part of the responsibility for chemical safety and effectiveness. As a best practice, buyers should compare supplier reports, request recent test data, and read product bulletins closely. Don’t just search for the highest number. Assess whether low or ultra-high purity is actually necessary for your use. Overspending for “absolute” purity rarely makes sense in non-critical settings.
Clear communication and regular checks—combined with a dose of skepticism—help build resilient processes. Stakeholders who keep purity and concentration front-of-mind dodge trouble, save money, and make science and industry safer for everyone relying on that bottle or barrel.
Why Storage Matters for Chemical Stability
In my time working alongside chemists in university research labs, poor storage led to more than one costly mishap. Take a bottle of 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate, for instance. This ionic liquid stands out for its use in catalysis, electrochemistry, and as a solvent for biomass transformation. Some labs overlook the details, but the right storage approach can keep costs down and experiments on track.
Keeping It Dry and Away from Air
Humidity and air both cause headaches. 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate attracts water. Open the bottle in a humid room, and you'll notice it absorbs moisture from the air. Water changes its properties, which ruins measurements and reactions. Storing it in a cool, dry spot pays off in the long run.
I learned quickly to reach for a desiccator, a sealed box filled with drying agents, after every use. Whether you have silica gel or another desiccant on hand, it buys peace of mind by pulling water away from the chemical. A tightly sealed amber glass bottle keeps the liquid safe from light and air. Certain imidazolium salts also break down in strong light. So, shelf placement matters—tuck the bottle away from windows or harsh ceiling lights.
Keep Temperatures Low, Not Frozen
I once made the mistake of popping an ionic liquid into the freezer, thinking colder was always better. In reality, 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate does better around 2–8°C, the temperature range of a standard lab fridge. Extreme cold can change its viscosity or crystalize it. At room temperature, especially in a tropical climate, the liquid will degrade faster. Store it in consistent, chilled conditions. That simple routine avoids unwanted surprises during research or industrial runs.
Clear Labeling and Regular Checks
Labeling isn’t just busywork—it prevents a lot of guesswork and wasted material. After opening a fresh bottle, writing the date keeps shelf life realistic. Most manufacturers recommend using this compound within two years of receipt, provided the original container stays tightly closed and conditions remain stable. If the color, smell, or consistency shifts, don’t risk trials with it. Years of trial and error have shown me that stretching the timeline for organics almost never pays off.
Avoiding Contamination
Cross-contamination in the lab sneaks up on even seasoned folks. Using only clean, dry pipettes and spatulas with this liquid keeps impurities out. Residues from other substances—especially acids or bases—can start unwanted side reactions. A few years ago, contamination resulted in failed tests and hours of detective work. That lesson showed me that a little extra caution saves chemicals and time.
Safe and Sensible Disposal
Many forget about disposal, but improper storage at end-of-life can create risks. Residual 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate isn’t household waste. My old lab established a system so these wastes end up in designated hazardous waste containers, avoiding sewer or landfill. Communities with robust systems protect workers and the environment from harm.
Room for Improvement
Better packaging with moisture indicators or tamper-evident seals could help large users. Improved training helps lab teams handle these specialty chemicals responsibly. Industry-wide, more facilities recognize the need for climate-controlled chemical storage. They invest because each spill or spoiled bottle costs more than a shelf or a new label.
The Essentials on 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate
Curiosity about specialty chemicals often starts with the hard data. For 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate, the molecular weight clocks in at 236.33 g/mol. Chemists tag this substance with CAS number 472477-32-2. These numbers aren’t just technical trivia—they shape everything from safe handling to research breakthroughs. Working in labs, I’ve seen people confuse compounds because they skipped a double-check on the CAS number. Mislabeling might not sound serious until you realize a sequence of tests just got built on the wrong foundation.
Why Exact Numbers Change Lab Life
Molecular weight matters a lot. In practice, it drives how to measure, dilute, and react a substance. Slip up here, and projects fall apart. I’ve watched chemists calculate reagents using the wrong weight and burn through weeks of effort fixing the fallout. Even a seasoned professional can fumble measurements without simple, accurate reference numbers. Sure, the CAS number seems like a dull string of digits, yet it saves hours digging through databases and makes global communication easier. If you use a casual name or shorthand, you risk the kind of confusion that can slow an entire team.
From Green Chemistry to Safer Workplaces
Many folks view ionic liquids like 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate as the next step for greener chemistry. They draw attention because they don’t evaporate like old-school organic solvents; as a result, they offer potential for lower toxicity and less air pollution. But nobody should take that as a free pass. Magic bullet thinking disappears fast in the lab, where trace contamination or unexpected byproducts always invite surprises. Clear molecular weights and true CAS numbers help people dig into toxicity, disposal, and purity data, raising safety for us all.
Details Build Better Chemistry
Too many research teams cut corners by relying on internet snippets or supplier brochures with half-complete specifications. In chemistry, incomplete data means wasted time and burned budgets. During my graduate years, suppliers sometimes sent over samples with mixed up CAS numbers or different salt forms. Sorting out the mess took extra research, extra controls, and sometimes repeating experiments. Nobody likes explaining delays caused by chasing the wrong compound through months of work.
Smart Habits for Safer and Smarter Science
Consistency trumps guesswork. Chemical manufacturers, researchers, and students all benefit from pulling molecular weights and CAS numbers straight from trusted databases. Tools like PubChem and Sigma-Aldrich catalogs help by cross-referencing updates and corrections, making science more reliable for both veterans and newcomers. Remembering each number, or at least knowing where to find it in a hurry, leads to sharper work and fewer safety incidents.
Building Trust through Transparent Reporting
Listing that 1-Ethyl-2,3-Dimethylimidazolium Ethylsulfate equals 236.33 g/mol and bears CAS number 472477-32-2 isn’t just box-ticking. It’s how professionals build shared knowledge and protect everyone from needless risk. Before anyone pours, stirs, or heats a flask, these details drive responsible, credible research—on the bench, on paper, and out in the wider world.