1-Ethyl-2,3-Dimethylimidazolium Tosylate: An In-Depth Perspective
Historical Development
Ionic liquids drew curiosity in the late twentieth century as chemists started searching for alternatives to volatile organic solvents. Streams of research followed the trail of imidazolium salts because these molecules can withstand heat and remain stable across a broad range of chemical environments. Normally, chemists wrestle with balancing a solvent’s reactivity with its safety and environmental profile. The idea of using 1-ethyl-2,3-dimethylimidazolium tosylate (often known as [EDMIM][OTs]) grew out of the same function-driven urge—a push to combine a strongly polar structure with low vapor pressure and good miscibility. After researchers noticed how certain cations such as imidazolium paired with bulky anions like tosylate (para-toluenesulfonate) performed in catalysis or electrochemistry, the popularity of this compound increased. Its synthesis became more routine as laboratories adapted old methods, swapped in less hazardous reagents, and improved purification techniques. The compound’s rise matches the growing reputation of green chemistry, safer laboratory practices, and exploration into new chemical frontiers.
Product Overview
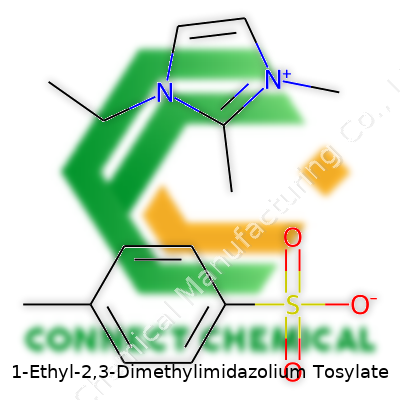
1-Ethyl-2,3-dimethylimidazolium tosylate stands out from earlier ionic liquids by offering a unique set of traits: high thermal stability, non-flammability and robust solvation ability for both organic and inorganic materials. With an imidazolium ring core modified at the 1 and 2,3 positions with ethyl and methyl groups, plus the tosylate counterion, this ionic liquid often appears as a slightly viscous, colorless to pale yellow liquid at room temperature. Suppliers frequently offer the product at research-grade purities, commonly above 98%. It ships in tightly sealed amber bottles to cut down water absorption and light exposure, since water changes its physical dynamics and UV can degrade sensitive chemical stocks. Many kits now include inert gas blanketing or moisture scrubbing sachets.
Physical & Chemical Properties
1-Ethyl-2,3-dimethylimidazolium tosylate typically displays a melting point around 40°C, with a decomposition temperature above 250°C. It carries a high ionic conductivity—often measured above 5 mS/cm at room temperature—far exceeding typical organic solvents. This ionic liquid resists hydrolysis and oxidation under standard laboratory conditions. Its density, usually about 1.12 g/cm³, makes handling straightforward; it sits between water and typical organic liquids. With good miscibility in a wide range of solvents (including water, dimethylformamide, acetonitrile, and toluene), EDMIM tosylate provides a very adaptable medium for catalysis, material synthesis, and analytical applications.
Technical Specifications & Labeling
Labels on bottles should clearly indicate the compound’s full IUPAC name and its most common synonyms: 1-ethyl-2,3-dimethylimidazolium p-toluenesulfonate, [EDMIM][OTs], or sometimes abbreviated as EMI[TsO]. The CAS number (1127755-99-6) guarantees traceability of batch, synthesis method, and purity. Certificates of analysis from reputable suppliers detail each batch’s moisture content, UV absorbance profiles, and, sometimes, residual halide or metal content, which matters if the product heads into electronics or catalysis research. Shipping requires compliance with standard hazardous material guidelines, since some authorities flag ionic liquids possessing strong oxidizing or reactive groups, though EDMIM tosylate’s profile falls toward the milder end. In the lab, bottles need storage below 30°C, away from chlorinated reagents and strong bases.
Preparation Method
Classic preparation begins by alkylation of 2,3-dimethylimidazole with ethyl bromide in anhydrous solvent, often under nitrogen atmosphere. Careful temperature control pulls up yields by cutting side-reactions. After the crude 1-ethyl-2,3-dimethylimidazolium bromide forms, it reacts with sodium tosylate (or an equivalent) in water or an alcohol. Ion-exchange occurs, yielding the final ionic liquid and a salt byproduct. Longer reaction times and improved purification through recrystallization or solvent washing help push purity. Techniques such as vacuum drying remove water, since hydrated salts behave differently in catalysis and conductivity. Researchers with experience in purification, especially those familiar with glovebox or Schlenk-line techniques, move through these steps with fewer complications and better batch-to-batch reproducibility.
Chemical Reactions & Modifications
1-Ethyl-2,3-dimethylimidazolium tosylate can take part in various template reactions as both solvent and reagent. The strong polarity and ionic nature let it dissolve a variety of metal salts or organic substrates, opening the door to organometallic catalysis, transesterification, and polymerization. Sometimes it acts as a phase transfer agent, moving inorganic anions across boundaries between organic and aqueous media. Its stability toward mild acids and bases makes it attractive in multi-step synthesis where solvent needs to survive frequent pH shifts. Chemists can adjust its properties through anion exchange: swapping tosylate with hexafluorophosphate, tetrafluoroborate, or dicyanamide shapes the physical and nucleophilic properties of the resulting liquid. Careful handling minimizes impurity buildup that can kill yield or catalysis rates; regular screening through NMR or ion chromatography keeps performance on track.
Synonyms & Product Names
Since many laboratories rely on chemical suppliers that use different naming conventions, this compound may also show up as 1-ethyl-2,3-dimethyl-1H-imidazol-3-ium tosylate, EMI Tosylate, or 1-ethyl-2,3-dimethylimidazolium para-toluenesulfonate. Product codes vary from vendor to vendor, but trustworthy catalogs always tie the label to the correct CAS number and provide the molecular structure alongside the name. Mismatches in labeling lead to headaches for procurement officers and experimentalists; always double-check bottle labels against primary literature.
Safety & Operational Standards
Working with ionic liquids such as 1-ethyl-2,3-dimethylimidazolium tosylate often poses fewer acute hazards than traditional organic solvents—no flammable vapors or aggressive volatility. Still, safety teams push for good ventilation, gloves, and splash protection because trace imidazolium compounds can irritate skin or mucous membranes. Tosylate salts occasionally trigger respiratory responses in sensitive individuals. If a spill lands on the benchtop, neutralizing and wiping up with plenty of water suffices for most incidents. Waste collection bins labeled for halogenated materials usually accept these spent or contaminated ionic liquids. Researchers tracking long-term exposure or waste management adopt the guiding principle: just because a liquid acts safer doesn’t mean regulations can slip.
Application Area
Interest stretches across major fields. Analytical chemists use this ionic liquid for extracting metal ions from waste streams or as an electrochemical electrolyte for high-temperature sensors. Materials scientists turn to EDMIM tosylate when fabricating conductive polymers or casting nanocomposites because of its plasticizing effect and stable ionic background. Synthetic organic chemists value its role as a green solvent in alkylation, cycloaddition, and rearrangement reactions—sometimes seeing big jumps in reaction rates or selectivity compared to the same experiments run in classic solvents. Life sciences groups dabble in using it as a stabilizer for enzymes, proteins or even drug delivery applications. The breadth of use points to an adaptability born of unique chemistry, and it breeds cross-talk among specialists in different labs.
Research & Development
Institutes keep asking how to push ionic liquids further—higher conductivity, broader thermal range, and lower toxicity often anchor those questions. EDMIM tosylate has become a staple in studies looking at battery technology, especially in non-flammable battery electrolytes. Research groups probe its use in supercapacitors, rechargeable systems, and direct methanol fuel cells, since the ionic framework boosts both safety and performance. On the synthesis front, teams optimize how quickly the compound can host metal-catalyzed reactions or phase-transfer events, sometimes hitting reaction rates that save hours or days over non-ionic setups. Universities want to squeeze more data out of existing compounds by coupling the liquid with nanoparticles, enzyme cascades, or flow chemistry hardware. Funding agencies, noticing the push for sustainable and less-wasteful chemistry, place value on projects using EDMIM tosylate as their backbone.
Toxicity Research
Ionic liquid safety reflects a mix of chemical intuition and real-world data. Toxicity studies for 1-ethyl-2,3-dimethylimidazolium tosylate often run in parallel with tests on similar imidazolium salts. Researchers expose cell cultures, aquatic animals, or even soil microbes to measured doses and monitor for enzyme inhibition, reproduction effects, or acute lethality. Many studies show low volatility keeps immediate inhalation risk low, but chronic effects—especially on aquatic systems—need careful monitoring since these organic ions do not degrade quickly in the environment. Animal studies sometimes flag mitochondrial effects at high doses, which raises a red flag about large-scale releases or lab practices that don’t contain waste. More data will help fine-tune regulatory guidelines and push manufacturers toward greener modifications where possible.
Future Prospects
With the global shift toward sustainability, chemists push for energy-efficient processes, recycling, and minimal emissions. EDMIM tosylate lines up well with these shifts. Battery manufacturers test its use in safer and longer-lived lithium and sodium systems. Chemical engineers rethink old batch processes, swapping in ionic liquids to either combine multiple steps or capture heat that would otherwise go to waste. As new regulations phase out hazardous aromatic solvents and persistent halogenated compounds, more producers adopt this family of ionic liquids to keep research progressing without hitting safety snags. The next wave likely includes more hybrid systems—ionic liquids connected to polymers, supported on solid scaffolds, or used in tandem with biological catalysts for green production lines. As teams learn from both success and mishap, the compound will help reshape the way scientists approach problems both big and small.
Behind the Strange Name: What Sets It Apart
Most people never hear about 1-ethyl-2,3-dimethylimidazolium tosylate. It’s a mouthful, for sure, but for those who do research, its value can’t be ignored. As someone who has spent hours puzzling over which solvent best fits a tough chemical reaction, I’ve noticed that materials like this ionic liquid keep showing up in papers—and not by accident.
Why Ionic Liquids Matter in the Lab
Ionic liquids shift the way scientists approach tough lab problems. Unlike water or most organic solvents, these liquids don’t evaporate much and they hold up under heat. 1-ethyl-2,3-dimethylimidazolium tosylate stands out for its steady performance during temperature cycling. With it, you let reactions run longer or hotter—no smoky lab, no glass boiler breaking under pressure.
Cleaner and Greener Reactions
Green chemistry has become more than a buzzword. Regulators—especially in the EU and California—push labs to swap out hazardous organics for safer alternatives. This ionic liquid rarely flies off into the air, making spills less hazardous and recycling simpler. One research group published data in ChemSusChem showing up to 80% reduction in volatile emissions when replacing classic solvents with imidazolium-based liquids during cellulose processing.
How It Boosts Industrial Work
Large-scale chemistry turns on practical details. Plants look for solvents that dissolve tough materials, work with strong acids, and don’t corrode equipment in a week. Here, 1-ethyl-2,3-dimethylimidazolium tosylate steps up. In dye-sensitized solar cell manufacturing, teams use this ionic liquid to dissolve both organic and inorganic chemicals, giving engineers a single solution instead of juggling half a dozen incompatible options. It’s also shown promise in battery research, where stable performance under charge-discharge stress helps new ideas get off the ground before companies pour in the big investment.
Challenges Don’t Disappear
Cost holds many labs back from jumping into ionic liquid projects. These compounds generally cost more than gallons of old-school solvents, so the pay-off must be real—not just theoretical. My own experiment budget took a serious hit swapping to newer ionic liquids, and I’ve seen colleagues limit use to smaller scales. To deal with this, several companies and universities now research recycling strategies. By recovering and purifying these liquids on-site, factories can slash long-term costs and cut waste streams, as highlighted in a 2021 study in Green Chemistry.
The Real-World Payoff
1-ethyl-2,3-dimethylimidazolium tosylate means better safety, cleaner air, and tough reactions with fewer worries. It also nudges researchers closer to less-polluting industry, which matters in any century. I’ve watched students spark new ideas when they realize this – fewer restrictions and more room to try new approaches. The pay-off sits not just in results, but in a mindset shift toward safer, smarter chemistry.
Understanding What’s Inside
Sometimes, the way a chemical compound comes together tells us a lot about its uses. Take 1-Ethyl-2,3-Dimethylimidazolium Tosylate. People working in labs spot its name often because it belongs to the ionic liquids family. These kinds of salts stay liquid under comfortable temperatures, so folks in research settings have begun leaning on them for tasks that water or oil just can’t manage. Getting a feel for its makeup helps explain why it plays such a useful role.
Breaking Down the Cation
The cation half of the story, 1-Ethyl-2,3-Dimethylimidazolium, comes from the imidazole ring. That’s a five-membered ring made up of three carbon atoms and two nitrogen atoms. In this structure, the first nitrogen connects to an ethyl group (meaning, two carbons lined up). Carbon numbers two and three both get a methyl group stuck on—just a single carbon apiece. You end up with a tight little ring that’s positively charged and not shy about mixing with a range of other chemicals, including some that water steers clear from.
The Power of the Tosylate Anion
Tosylate doesn’t show up as much in daily life, but its shape and attitude matter in chemistry. Think of it as a benzene ring (that’s six carbons in a neat hexagon with hydrogen filling the gaps) with a methyl group anchored to one spot, making it toluene. Now stick a sulfonate group (SO3-) on as well. Sulfonates bring in a hefty negative charge, balancing out the cation perfectly, making it stable in a bottle or a beaker.
Why Ionic Liquids Matter
I come at this as someone who’s worked at a lab bench and in classrooms where new solvents show up every few months. Regular solvents, like water or acetone, have their strengths, but the imidazolium-based ionic liquids kicked down doors for people chasing tough-to-solve problems. These salts barely evaporate, helping researchers cut down on toxic fumes—no one misses the haze that comes from organic vapors. They dissolve metal salts, strong organic compounds, and polymers, making strange reactions possible. The imidazolium ring’s stability, mixed with the right anion like tosylate, lets chemists fine-tune their work rather than fighting the solvent at every step.
Challenges and Paths Forward
It would be off-base to skip the downsides. Ionic liquids can cost a fair bit, and some versions break down under too much heat or after repeated cycling. Long-term effects, especially waste and recycling issues, stay a worry. Companies and labs can press for “greener” designs—swap out the aromatic anions for simpler, more biodegradable ones, or test new cation patterns that hold up better under stress. If manufacturers share more about their methods and set up clear recycling systems, a wider group of researchers could work safely and cheaply.
Looking at the Future
Chemists see these engineered structures, like 1-Ethyl-2,3-Dimethylimidazolium Tosylate, as more than a lab curiosity. Better solvent systems will push green chemistry and cleaner manufacturing forward. If more people understand what these chemicals look like—how their funky shapes tie into real uses—they can make sound choices, whether planning safer experiments or managing waste at the end of a process. Getting familiar with structure pays off, not just in the lab, but in building safer habits for everyone.
Understanding the Chemical
The world of ionic liquids often feels confusing. I remember staring at strange names in my old undergraduate textbooks and wondering who decided molecules needed so many syllables. Names like 1-ethyl-2,3-dimethylimidazolium tosylate can intimidate, but the underlying chemistry tells a simple story. This compound belongs to a family known as imidazolium-based ionic liquids. These have picked up steam for their ability to dissolve a surprising range of materials.
What Guides Solubility?
In chemistry, “like dissolves like” stays true more often than not. Imidazolium salts, by nature, like polar environments and often find a home in water. Still, the business end of this chemical, the tosylate anion, offers a bit of drama. Tosylate, being larger and somewhat less hydrophilic than something like chloride, shifts the solubility needle a little. Adding ethyl and methyl groups to the imidazolium cation also increases hydrophobic character, setting up an interesting competition between hydrophilic and hydrophobic properties.
The Reality with Water
I once tried dissolving a small batch of an imidazolium tosylate in water during a lab rotation. The results came as no surprise: water handled the solution smoothly. This echoes what much of the literature confirms. Most 1-ethyl-2,3-dimethylimidazolium salts, including the tosylate variant, blend into water with minimal fuss, though their behavior changes as the amount of organic decoration increases.
Studies have measured solubility values in excess of 100 g/L for related imidazolium ionic liquids. Sources like the Journal of Chemical & Engineering Data frequently report favorable solubility, especially for tosylate salts with relatively short side chains. Imidazolium-based liquids like the one in question enter solution clear and stable—hardly the case with more grease-loving ionic liquids.
Why This Solubility Matters
So, the stuff dissolves well in water. Why should anyone care? From a chemical engineer’s perspective, this opens up the field for green chemistry. Water-soluble ionic liquids provide alternatives to traditional organic solvents, which contaminate waterways or give headaches from their fumes. When synthesis happens in water, reaction clean-up gets easier, waste drops, safety rises.
I’ve seen researchers use water solutions of imidazolium tosylates to pull metal catalysts out, run enzyme reactions at room temperature, and clean lignin from wood chips without wrecking the fibers. Water solubility here becomes more than a curiosity—it supports new ways to extract, purify, and process chemicals. No need for heavy-duty fume hoods or flammable hazards.
Hurdles and How to Tackle Them
Every advantage has a shadow. High water solubility also means these chemicals can end up in the environment. They don’t always break down quickly. Ionic liquids often act as persistent pollutants unless properly collected and handled. Research groups should lean on more thorough risk assessments and practice closed-loop recycling, keeping chemicals out of rivers and groundwater.
Stricter disposal rules help prevent contamination, while molecular tweaks—like swapping the tosylate for something more biodegradable—bring hope for safer future generations of these chemicals. For universities and industry labs, training matters: know the properties, respect the risks, keep water clean.
Final Thoughts
Solubility often gets brushed aside as trivia. In fact, it shapes the options chemists have for design and cleanup. Here, the answer is clear: 1-ethyl-2,3-dimethylimidazolium tosylate and water get along well. Smart use can help labs and factories move forward. Wise handling will keep that progress safe.
Getting Real About Handling Ionic Liquids
1-Ethyl-2,3-Dimethylimidazolium Tosylate, a specialized ionic liquid, pulls a lot of attention from chemists because of its useful properties in catalysis and synthesis. Even so, handling it without thinking much about safety can turn things sour in the lab.
Understanding the Risks
This compound doesn’t have the volatility of many organic solvents, but that doesn’t excuse reckless behavior. Many folks assume liquids that don’t give off strong odors or burn quickly are “safe.” That line of thinking misses the risks at hand. Some ionic liquids cause irritation if they touch skin or get in the eyes. Inhaling dust or fumes—or accidental splashes from a clumsy pour—invites trouble. Take it from someone who’s spent enough afternoons rinsing off after contact with odd chemicals: prevention beats the sting of mishaps every time.
Protect Yourself From Contact
Always gear up before working with this stuff. Nitrile or neoprene gloves hold up better than simple latex if spills happen. A lab coat with close-fitting sleeves keeps arms covered. I’ve seen too many stains on someone’s favorite shirt because they “didn’t expect mess.” Splash goggles bring more peace of mind than regular glasses. Face shields might sound like overkill, but if you’re transferring bigger volumes—say, filling bottles or prepping for a reaction—they add an extra layer between your face and unexpected splashes.
Good Habits While Working
Ventilation beats holding your breath. Work with open bottles in a fume hood or under a ventilated enclosure. This keeps airborne particles and any accidental acid vapors out of your breathing zone. Folks sometimes forget about labeling and tracking amounts. Clear labels, from opening to disposal, help avoid confusion, especially as bottles and samples pile up. Having a lab mate check over your handling routines builds good habits and keeps mistakes from slipping by.
Spill and Exposure Response
No one wants a spill, but planning for it keeps panic at bay. Spills don’t get smaller the longer you wait. Absorbent pads or spill kits with granules designed for non-volatile organics work well here. Shoveling up a mess with the wrong material spreads contamination and can make things worse. Properly disposing of cleanup waste in dedicated chemical bins and never down the drain helps keep the local community’s water cleaner.
Storage Matters Too
Ionic liquids like this one benefit from tight-sealing bottles made of glass or compatible plastic. Humid conditions degrade quality and introduce risks of unexpected reactions. A cool, well-ventilated spot far from acids, oxidizers, and open flames does the trick. In shared spaces, it pays to store this compound separate from unrelated solvents and corrosives.
Disposal Takes Attention
Ditching leftovers by dumping them into a sink isn’t just lazy—it’s potentially illegal and certainly bad for the environment. Designate a bottle or can for ionic liquid waste, then arrange regular collection through a chemical waste management company. Municipal labs often work with approved disposal services. Taking time to do things right pays off later, both legally and for everyone’s safety.
Learning From Each Use
Each time you handle a new bottle, review current safety data and material safety sheets from reliable sources like Sigma-Aldrich or the manufacturer. If uncertainty creeps up, pausing to double-check a procedure or ask a colleague keeps standards high. Mistakes rarely happen because of the “big stuff”—it’s skipping those small, routine checks that lets risk sneak in.
Responsible handling supports the whole lab, boosts confidence in research, and leaves fewer surprises behind for the next person reaching for the same bottle. With smart habits and steady awareness, even specialized chemicals like 1-Ethyl-2,3-Dimethylimidazolium Tosylate fit safely into daily work.
Why Paying Attention to Storage Matters
On the lab bench, every chemical tells its own story. 1-Ethyl-2,3-Dimethylimidazolium Tosylate often shows up in research involving ionic liquids, electrochemistry, or material science. Folks handling it—students, researchers, or plant staff—know small oversights can ripple into something bigger. Problems like moisture creeping in, cross-contamination, or temperature swings aren’t just minor speed bumps. They can break an experiment, or worse, they can endanger health. I’ve seen perfectly good chemicals lost because someone popped them into a random corner, thinking there’d be no consequence. The reality? There always is.
The Enemies: Water, Heat, and Air
Ask around any lab and you’ll hear similar stories—humidity always finds its way in. Even a cap not twisted tight can mean a ruined batch by the next morning. This compound stands sensitive to water, soaking it up from the air if allowed. That’s not just a hassle for calculations or mixing accuracy. It can spark unexpected reactions or degrade the substance itself. Working on a project once, I saw the difference between a sample carefully kept in a desiccator and one left in the open air; the bad one clumped, refused to dissolve, and gave inconclusive results. No shortcut fixes that.
Best Practices from the Front Line
Let’s talk storage solutions that actually work. Keep this compound in a tightly sealed, chemically resistant container—glass with a screw cap seems ideal. Avoid plastic jars that can let solvents or air sneak through. Hold onto the original packaging if you can, as manufacturers pick materials with the chemical’s quirks in mind. After every use, make it a habit to reseal and label the jar. I’ve learned not to trust memory for what’s in unmarked bottles.
Pick a cool, dry place away from sunlight or sharp temperature shifts. Refrigeration can help, especially if the room tends to run warm, but only if moisture can’t sneak inside the fridge or container. A desiccator filled with drying agents offers another layer of protection. Don't let the drying beads run out without swapping them for fresh ones—once saturated, they do nothing.
Handling with Care: Safety Isn’t Optional
Lab culture shapes storage habits. Set the example by wearing gloves and goggles during every transfer. Not every spill turns catastrophic, but the chemical shouldn’t touch skin or eyes. Even inhaling vapors or dust can cause irritation. I always make a point to wipe down benches and check for leftover residues—these steps protect the whole team.
Storing near incompatible chemicals kicks up the risk. Acids, strong bases, and oxidizers don’t belong near this compound. Mixing them accidentally could cause weird reactions—once, a misplaced bottle nearly ruined an acid cabinet. Double-check shelving and storage charts. Segregate chemicals by family, and never cut corners just to save space.
Reducing Waste and Costs Through Smart Storage
Replacing spoiled chemicals hits the budget and delays progress. Thoughtful storage stretches every purchase, shrinks hazardous waste, and keeps experiments on track. Training every new team member in these habits makes a difference. Tidy records help catch mistakes before they snowball. Smart labs run like this: they respect every bottle and the energy poured into obtaining it.