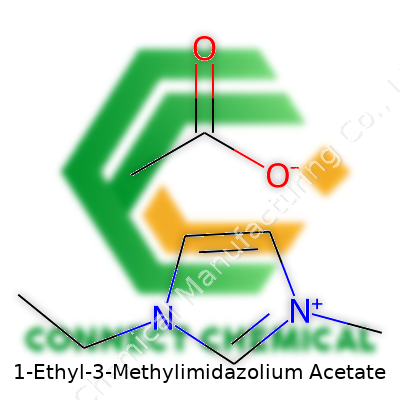
Digging into 1-Ethyl-3-Methylimidazolium Acetate: Beyond the Basics
Historical Development
Ionic liquids once sounded like a moonshot—a class of compounds barely known beyond academic circles. The breakthrough with 1-ethyl-3-methylimidazolium acetate (EMIM Ac) didn’t arrive overnight. Its roots trace back to the push for greener, alternative solvents in the late twentieth century. Back in the 1970s, researchers faced headaches caused by high volatility in traditional solvents, especially those used in cellulose processing and other tough separations. Over time, the clever modification of the imidazolium cation and anion combinations led to EMIM Ac, a star for both laboratory and industry. Seeing EMIM Ac replace hazardous solvents in dozens of applications felt like switching from leaded gas to electric. The surge in research around the early 2000s pulled EMIM Ac into the spotlight as a model system for sustainable chemistry.
Product Overview
Lab technicians and industrial chemists prize EMIM Ac thanks to its unique qualities. This clear to pale yellow liquid doesn’t show high vapor pressure at room temperature. The reason EMIM Ac turns heads comes from how well it dissolves cellulose, making it a natural fit for the biofuel and biopolymer sectors. Its non-flammability and ease of recycling lower hazards compared with old-school organics. Companies market EMIM Ac as an “advanced solvent” but that’s underselling it—it’s a true enabler for innovations where other liquids don’t measure up.
Physical & Chemical Properties
What sets EMIM Ac apart? Its density usually lands around 1.10–1.12 g/cm³ at 25°C, hinting at how it packs molecules tighter than water. It stays stable from –20°C through nearly 80°C, so no need to coddle it with careful storage. Unlike many other acetates, EMIM Ac absorbs water so strongly that any open container soon picks up extra weight. That makes it tricky in moisture-sensitive work but ideal for certain catalysis reactions. On the chemical front, EMIM Ac barely reacts with most metals, supporting a wide palette of uses that metallic-catalyzed syntheses. The ionic structure resists both oxidation and reduction under normal conditions.
Technical Specifications & Labeling
Commercial suppliers list EMIM Ac with a typical purity north of 98%, and water content usually kept under 0.1%. Clear labeling details include the CAS number (143314-17-4), batch lot, manufacturer, date of production, and expiry information. Many suppliers now add certificates of analysis so buyers know impurities won’t trip up sensitive applications like precision analytics or pharmaceutical research. It arrives in sealed glass or plastic bottles designed to keep out air and water, since a stray whiff of humidity could complicate downstream chemistry.
Preparation Method
The synthesis story for EMIM Ac tracks the evolution of green chemistry. The process starts with the alkylation of imidazole to yield 1-ethyl-3-methylimidazolium chloride, using either ethyl chloride or dimethyl sulfate. Next, anion exchange with sodium acetate transforms the chloride salt into the acetate version. This two-step approach avoids messy byproducts and doesn’t need high-temperature reactors. People with experience in small-scale production know the importance of careful purification to strip away unreacted starting materials, which could interfere during cellulose dissolution or bio-conversion work.
Chemical Reactions & Modifications
EMIM Ac proves more than just a solvent—it steps into reactions as both a participant and a stabilizer. Its acetate anion activates cellulose for complete swelling and dissolution, a challenge unmatched by most liquids. In peptide synthesis, EMIM Ac enables coupling reactions without the need for activating reagents that often create unwanted side products. Certain modifications on its imidazole ring yield task-specific ionic liquids to enhance solubility or shift polarity for special situations. Catalytic hydrogenations, separations, and even electrochemical transformations all benefit from EMIM Ac’s ability to tune reactivity and suppress undesired side reactions. Researchers found its low volatility crucial for the recovery and reuse in cyclic enzyme processes—cutting down not only waste but also operating costs.
Synonyms & Product Names
Despite the mouthful official name, EMIM Ac pops up in catalogs under several labels: 1-ethyl-3-methylimidazolium acetate, EMIM acetate, [EMIM][Ac], and even Imidazolium acetate. Some sources swap minor naming conventions—ethylmethylimidazolium acetate shows up in chemical literature. For anyone double-checking substances before adding to a mixture, keeping tabs on these aliases is crucial to avoid confusion and costly mistakes.
Safety & Operational Standards
Handling EMIM Ac shouldn’t get anyone’s adrenaline pumping, but a few ground rules apply. Even though it doesn’t catch fire easily, it causes skin or eye irritation after prolonged contact. Lab workers need gloves and safety goggles as a given. Open-air evaporation won’t poison the air, but any spills cause sticky floors and residue that can attract water fast. Good ventilation and closed-system processing lower risk. Disposal guidelines require professional waste streams since breakdown products are still under study; dumping down a drain won’t fly with regulators. Documentation about safe storage and emergency cleanup appears both in supplier safety data sheets and lab manuals.
Application Area
Cellulose processing still leads the way—any plant-based fiber treatment operation can tell stories about switching out sulfur-rich solvents for EMIM Ac and seeing efficiency leap. But EMIM Ac extends much further: its ability to dissolve biopolymers brings it into biomaterials, plastics, and dye production. Certain fuel cell projects use EMIM Ac as a supporting electrolyte with robust performance under load. In pharma research, EMIM Ac helps extract and purify complex natural products when supply is tight. As biorefineries scale up, EMIM Ac’s role widens, from raw biomass deconstruction to supporting enzyme catalysis for fine chemicals. Academic groups even test EMIM Ac to capture CO₂, offering a peek at possible roles in carbon sequestration.
Research & Development
Each year brings new studies into EMIM Ac’s strengths and boundaries. Labs all over the world publish methods to recycle and reuse EMIM Ac, aiming for true circularity in biorefining. Materials chemists run trials blending EMIM Ac into polymer composites for flexible batteries, medical devices, and bioactive films. Some research teams develop solid-state forms and ionic gels, breaking traditional solvent molds entirely. Long-term environmental studies follow EMIM Ac in soil and water systems, checking for dead ends that could create unforeseen risks. Conferences always feature panels sharing lessons about cost reduction, alternate manufacturing routes, and greener disposal pathways.
Toxicity Research
Concerns about EMIM Ac’s fate in water and soils continue to motivate lab tests and field studies. Early toxicological assessments show relatively low acute toxicity to mammals by ingestion, but long-term effects and chronic exposures still need close monitoring. Studies in aquatic settings raise red flags about EMIM Ac’s impact on some fish and microorganisms, especially at high concentrations from heavy industrial use. Bioaccumulation appears limited, but breakdown pathways in the environment remain an active area for research. Proper handling, storage, and disposal play a key role in reducing accidental releases that might upend local ecosystems. Regulatory frameworks now urge upstream risk assessment and continuous monitoring.
Future Prospects
EMIM Ac’s story has just begun. Ongoing improvements in synthesis could trim costs and move away from non-renewable feedstocks. Researchers test functionalized derivatives for advanced separations in battery recycling and rare earth recovery. As biorefineries grow bigger, demand for robust, recyclable solvents will rise—EMIM Ac holds strong promise to fill that gap. Interdisciplinary collaborations between chemists, environmental scientists, and engineers push to unlock new pathways where EMIM Ac not only replaces old solvents but unlocks new reactions and materials. Building on today’s knowledge, tomorrow’s discoveries could push EMIM Ac into roles nobody pictured a decade ago, all while keeping one eye on safety and sustainability.
The Chosen Liquid for Green Chemistry
If you walk through a modern chemistry lab, you’ll spot all sorts of bottles and flasks. Among those, 1-ethyl-3-methylimidazolium acetate stands out. Scientists love to call it “EMIM Acetate” for short. I first bumped into this clear liquid while digging into sustainable ways to break down plant waste. Turns out, EMIM Acetate has flipped the script for anyone looking to make biofuels, new kinds of biodegradable plastics, or cheaper pharmaceuticals.
The Secret Behind Dissolving Biomass
Plant material, called biomass, locks up a ton of energy, but nature bundles it tight with cellulose, hemicellulose, and lignin. Most solvents throw in the towel trying to untangle these. EMIM Acetate works like a smart key: it goes after those tough hydrogen bonds in cellulose and pulls them apart. Instead of harsh acids or explosive heat, you pour EMIM Acetate over wood chips or straw, and the fibers start to loosen. Mills and biorefineries have jumped on board, hoping to squeeze more fuel and chemicals from corn stalks and wheat straw. According to a study in Green Chemistry, this ionic liquid can dissolve nearly 25% more cellulose than older solvents, lowering both time and energy costs.
Cleaner, Greener Processing
Today’s factories get dinged for using toxic chemicals and lots of water. EMIM Acetate doesn’t evaporate into the air, so workers aren’t breathing in nasty fumes. Recycling options keep popping up too. After the process finishes, operators add water or safe salts, and the cellulose falls out again, leaving the EMIM Acetate ready for another cycle. Waste adds up quickly in most industries. By letting chemists reuse their solvent multiple times, this new technology isn’t just clever—it’s cheaper and leaves less mess.
Opening the Door to New Materials
Textile makers look for smoother, stronger fibers. EMIM Acetate lets them spin plant cellulose into new fabrics, nearly as soft as silk but made from re-grown crops. I’ve seen this up close: companies in Europe now use ionic liquids to turn beechwood or bamboo into fibers for shirts, dresses, and even medical bandages. Since EMIM Acetate holds onto cellulose chains instead of chopping them up, the finished threads don’t fray as fast and stay gentle on skin.
Room for Improvement
EMIM Acetate isn’t perfect. It carries a decent price tag, and if big spills reach rivers, aquatic critters could run into trouble. Manufacturing the ionic liquid itself often calls for tight controls. Researchers are already testing ways to make it cheaper and safer. Some look to renewable sources or greener manufacturing steps. Labs are racing to engineer EMIM Acetate mixtures that dissolve cellulose even faster, or handle tougher feedstocks like yard trimmings.
The Road Ahead
As the planet hunts for better answers to plastic pollution and reliable biofuels, 1-ethyl-3-methylimidazolium acetate makes its case one reaction at a time. My own experience tells me that new chemistry tools only take root if they make both environmental and business sense. Right now, EMIM Acetate is helping to close that gap, nudging companies away from old, dirty solvents toward processes that might finally live up to the “green” in green tech.
A Closer Look at This Chemical
Many folks working in labs or around specialty solvents have probably seen 1-Ethyl-3-Methylimidazolium Acetate on supply shelves. This ionic liquid gained attention as a greener replacement for certain harsh solvents. Tasks like dissolving cellulose or extracting plant compounds have become less daunting thanks to this compound. But any substance with scientific buzz deserves a reality check—how safe can it really be for the average worker?
What the Science Says About Safety
According to up-to-date safety data sheets and peer-reviewed studies, 1-Ethyl-3-Methylimidazolium Acetate does not explode or ignite easily under normal conditions. That’s a step up from some solvents that need locked cabinets and constant monitoring. Even so, the lack of flames doesn't mean it belongs anywhere near bare skin. Handling barehanded or skipping eyewear brings real risks: skin redness, eye irritation, and, after extended or repeated exposure, possible chemical burns. Breathing in its vapors or mist is a bad idea since it may irritate the airway. Several toxicological reports flag it as harmful if swallowed or if someone breathes in enough vapor.
Personal Experience with Lab Protocols
After years working in research spaces, following standard chemical hygiene saved me from trouble countless times. For anything unfamiliar, a quick skim through the safety data sheet beats making a wrong move. Railroading ahead with only basic gloves or skipping splash goggles could put health on the line. In the real world, mistakes pile up around issues like transferring or disposing of ionic liquids, because they don’t float off into the air or degrade easily. Proper lab habits like always using chemical-resistant gloves and good ventilation matter just as much for this “safer” solvent as with its more hazardous cousins.
What Are the Long-Term Risks?
Recent years brought out more careful lab animal studies. Some reports note that long exposures to 1-Ethyl-3-Methylimidazolium Acetate can skew organ weights or cause mild toxicity. Results look less scary than old-school organic solvents, but safety officers at responsible companies still classify it as hazardous. Its slow breakdown in nature is another sticking point. If this substance leaks into the water table or gets dumped without care, it can last for years, with unknown effects on fish and soil bugs.
Smarter Handling Practices
Fume hoods and chemical splash goggles never go out of style. For people with sensitive skin or breathing issues, wearing double gloves and using local exhaust helps prevent issues down the line. On large-scale or industrial projects, having real spill containment and specific disposal plans matters, since flushing this chemical down the drain can cause headaches at wastewater treatment plants. If a spill does happen, wash any affected skin with lots of water and take off contaminated clothing right away.
Solutions That Work
Training stands out as the simplest line of defense. New lab members or process operators need real hands-on time learning safe transfer and cleanup steps, not just a quick briefing. Facilities can invest in digital sensors that keep tabs on air quality and alert workers to leaks, as small drips add up over months. Partnering with certified chemical waste handlers limits the risk of environmental mishap. As the green chemistry movement pushes more ionic liquids into common use, no shortcut protects better than clear rules and daily vigilance. Taking short-cuts can damage careers and put lab teams in the news for all the wrong reasons.
Why the Right Conditions Matter
Plenty of folks in labs or factories have handled 1-Ethyl-3-Methylimidazolium Acetate—often called EMIM Acetate. This ionic liquid has a reputation for versatility, especially in green chemistry and cellulose processing. Still, like any specialty chemical, EMIM Acetate asks for a bit of respect when it comes time to store it. Letting standards slip can mean wasted product, health risks, or worse. I’ve seen those risks firsthand: once, a colleague’s careless approach led to cross-contamination and a sticky, expensive mess. That experience sticks with me every time I seal up a drum or refill a vial.
The Main Enemies: Moisture, Air, and Heat
EMIM Acetate draws water out of the air. I’ve lost count of the times a bottle, left open too long, turned syrupy. So, water-tight, air-tight containers prove crucial. Glass bottles with ground-glass stoppers do a solid job, as do high-quality polyethylene bottles with proper seals. A desiccator isn’t overkill for this compound—silica gel packs or similar drying agents pay for themselves in product life.
Heat speeds up the trouble. Most manufacturers recommend keeping EMIM Acetate below 30°C—better yet, room temperature or a little cooler. Simple shelving in a shaded, indoor spot works, as long as it’s away from radiators, windows, or equipment that tends to warm up the room. Unneeded refrigeration brings its own headaches, like condensation, so that’s rarely my first choice unless things get really warm.
Oxygen’s a quieter threat. Ionic liquids stay stable with moderate oxygen, but for long-term storage, some professionals flush their containers with nitrogen. That’s a practice I only use when I’m working with large volumes—nothing like watching pricey chemicals slowly yellow with age to ruin your day.
Labeling and Safety Principles
Accurate labeling keeps things running smoothly. I record the date opened, the source, and any batch info right on the bottle. That log helps with traceability—a must if anything ever goes wrong. Chemical goggles and gloves always go on before handling EMIM Acetate. Even though it’s less volatile than many solvents, spills feel sticky and can irritate exposed skin.
If the compound does spill, I grab absorbent pads and keep the area ventilated. I’ve noticed that colleagues sometimes underestimate the slip risk—mopping up immediately saves a lot of dangerous near-misses.
Disposal Practices and Accidental Exposure
No one likes dealing with chemical waste, but safe disposal matters. The local hazardous waste service takes old EMIM Acetate; pouring it down the drain just creates headaches downstream. Special waste containers and following local rules keep the workplace safe and environmentally sound. In case of skin contact, washing with soap and running water remains the best move. If an eye splash happens, full irrigation and medical help come next.
Stronger Culture Around Storage
Each chemical brings its own quirks, and EMIM Acetate stands out for demanding dryness and coolness but not being particularly volatile or combustive. Training everyone—newbies and veterans—makes a difference. Regular reviews of safety data sheets, hands-on practice with containers, and quick reporting of leaks or odd smells set up a safer, more organized lab or plant. Solid habits beat high-tech solutions, in my book, every time.
Understanding the Route From My Own Lab Days
I’ve spent countless hours in chemistry labs, sleeves rolled up, beakers in hand, and the methods for making ionic liquids always stood out because you’re juggling both safety and unpredictability. The synthesis of 1-ethyl-3-methylimidazolium acetate, or EMIM Ac, tends to follow a two-step dance, one that reveals a lot about how careful planning makes a real difference in chemical manufacturing.
The Straightforward Approach
This compound starts its journey with two main ingredients: 1-methylimidazole and an alkylating agent, usually ethyl chloride. In my view, this first step teaches a basic but crucial lesson: simple chemicals can react, but the trick lies in getting the conditions just right. Directly mixing 1-methylimidazole and ethyl chloride in a controlled environment produces 1-ethyl-3-methylimidazolium chloride. You usually see scientists using solvents that can handle some mess—think acetonitrile or even dichloromethane. The mixture needs to stay cool, often with a fume hood running; ethyl chloride isn’t kind to careless hands or unprotected eyes.
The Ion Exchange Step Everyone Remembers
Converting the chloride salt to the acetate form stands out as the turning point. Most chemists I’ve worked with use metathesis to swap the chloride for acetate. Pour in an excess of sodium acetate, usually dissolved in water, and stir the mix with what I started to call “chemist patience.” The reaction between 1-ethyl-3-methylimidazolium chloride and sodium acetate gives the coveted EMIM Ac and sodium chloride as a byproduct. At this stage, you want the sodium chloride out of the way, so you filter or wash repeatedly. I remember the endless rounds of wash-and-settle at the bench, always aiming for a pure, undisturbed ionic liquid layer.
Quality and Purification: Why It Matters
A good ionic liquid isn’t just about “getting a yield.” Trace impurities, especially leftover chlorides or water, make processes unpredictable. Back during a project on cellulose dissolution, any salt residue could ruin the experiment, costing days of work. Most protocols call for treating the product under vacuum at mild temperatures, helping to chase off water. High vacuum drying transforms the product from a viscous mix to a crystal-clear ionic liquid, ready for testing. Lab reports confirm that, with the right attention, the acetate ion offers strong dissolving power for cellulose and biomass, thanks to its interaction with hydrogen bonds.
Challenges in Scaling and Safety
Lab syntheses scale up with bigger pots and more ambition, but risk climbs fast. Ethyl chloride, for example, brings a flashpoint risk, and the process generates gases you’d never want to breathe. Strong training guides every step—no shortcuts allowed. Labs follow EPA and OSHA guidance; industrial setups rely on enclosed reactors and proper scrubbing systems. I’ve seen scaling go sideways for teams rushing purity, only to lose the entire batch. Only tight process controls and real respect for safety keep things on track.
The Path Forward
EMIM Ac and other ionic liquids unlock advances in green chemistry, especially in cellulose processing and catalytic reactions, but lessons from hands-on work suggest that cleaner, more efficient syntheses remain a work in progress. Manufacturers and research labs need to keep tightening the process, minimizing energy and solvent use, and ensuring safer conditions for everyone. Synthesizing EMIM Ac isn’t glamorous, but the right attention to detail lets this molecule show its true value in science and industry.
Importance of Stated Purity for Researchers and Industry
No one dives into a lab project or gears up for a production run with chemical guesswork. Purity matters, especially with a compound like 1-Ethyl-3-Methylimidazolium Acetate (EMIM Ac), which features in everything from cellulose processing to battery development. In my own experience, a shipment labeled “99% pure” has calmed a lot of nerves just knowing that side-reactions won’t catch me off guard. It’s standard to see 99% or 99.5% purity listed from most reputable chemical suppliers. That number gives scientists and engineers a fighting chance against contamination—and keeps projects on track, budgets intact.
Testing Methods Behind the Label
Purity labels don’t come from trust alone. They usually come from a mix of spectroscopy (NMR, FTIR) and chromatography (HPLC, sometimes GC). The clearer the spectral fingerprint, the more reliable the sample. For EMIM Ac, water content gets checked by Karl Fischer titration because moisture, even at low levels, can throw off viscosity or trigger unwanted reactions. In a cellulose dissolution experiment I worked on, a slightly elevated water level in ionic liquids meant streaky, inconsistent results. A percentage point here can cost you days in the lab. Suppliers willing to show method details and batch-specific Certificates of Analysis make a big difference.
Common Impurities and Their Effects
Most batches come seasoned with trace imidazole, residual acetate, or leftover solvents. Even tiny bits can skew results: imagine chasing down an unexpected melting point only to learn the batch had a splash of methanol not listed on the spec sheet. It’s annoying, costly, and in a manufacturing environment, that mishap can scale up to lost product or, worse, process shutdowns. That’s why knowing what shows up below 1% matters. Working in a start-up setting, off-the-shelf stock never cut it without a fresh test—especially when scaling from pilot to small-production runs.
Specification Sheet Details Worth Attention
The best spec sheets don’t bury details in jargon. They highlight assay percentages, water content (often under 0.2%), color (pale yellow or colorless for a reason), and physical data like melting point (typically below room temp) and density. Also, don’t forget to check for listed metal ion content, since transition metals mess with EMIM Ac’s stability in catalytic applications; more than trace amounts can ruin a catalyst’s day. When a spec sheet lists low chloride, iron, and sodium, headaches later on are a lot less likely.
Solutions for Users: Testing and Communication
No one likes surprises—least of all with specialized chemicals. Ordering sample-sized amounts and running a quick NMR or water check in-house can catch problems early. For scale-up, asking your supplier for recent analysis isn’t just smart—it’s responsible. There’s value in suppliers who welcome transparency, send fresh results, or allow lot-specific re-testing. If the supplier balks at questions, time to look elsewhere.
The Takeaway: Purity Pays Off
Trying to save a few dollars by accepting “good enough” specs can backfire hard. Taking the time to check, confirm, and communicate expectations makes every stage—research, pilot, or full-scale—run smoother. Based on past headaches from glossed-over impurity details, I’d stake a project’s success more on honest specs and open communication than a rock-bottom price.