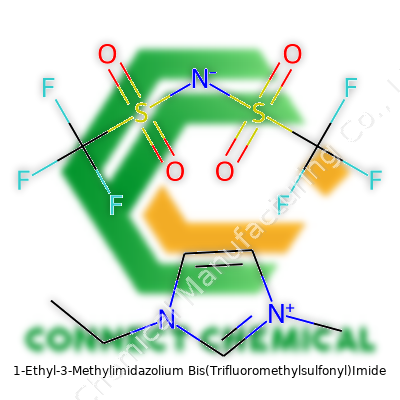
1-Ethyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide: A Closer Look
Tracing the Historical Journey
Chemists searching for cleaner, more adaptable solvents have often looked past the usual suspects. Old-school solvents dominated laboratories for decades, but environmental pressure built up. That pressure sparked interest in ionic liquids, substances that wouldn’t simply evaporate into the air and pollute the workspace. 1-Ethyl-3-Methylimidazolium bis(trifluoromethylsulfonyl)imide, also called [EMIM][TFSI], came out of this wave of innovation in the late 1990s and early 2000s. Its story is tied up with the bigger hunt for green chemistry solutions and safer electrochemical applications. Scientists put it through its paces, measuring its traits and handling as an alternative to volatile organic cocktails. The drive behind this research grew out of personal worry about lab safety, as anyone who has spilled solvents knows how quickly the air becomes unbreathable.
Getting to Know the Product
EMIM TFSI looks like a clear to pale yellow liquid at room temperature. Its lack of smell stands in sharp contrast to harsh, eye-watering cleaners crowding chemical benches. For a technician, picking EMIM TFSI over older solvents often means less coughing, fewer headaches, and easier clean-up. The imidazolium backbone gives it chemical stability. Its structure comes from a five-membered ring bearing the ethyl and methyl groups, balanced with the perfluorinated sulfonyl imide anion. This formulation allows the compound to work where most salts solidify, giving it some real flexibility especially in battery work and advanced synthesis. Those who have grown tired of weighing and mixing traditional solid salts will find pouring EMIM TFSI from a bottle refreshingly straightforward.
Understanding Physical and Chemical Properties
Anyone handling EMIM TFSI must learn its quirks. With a melting point around -16°C, it stays liquid in cold rooms. Its boiling point rises above 400°C under atmospheric pressure, meaning it won’t vanish on a hot day at the bench. The viscosity sits lower than many ionic liquids, floating around 32 cP at 25°C, so it moves easily in pipettes and spreads quickly over surfaces. Electrical conductivity remains high thanks to its mobile ions; values reach up to 10 mS/cm in the lab, making it a draw for folks wiring up experimental batteries. It dissolves a surprising range of organic and inorganic compounds, which cuts down on the need to keep dozens of specialty solvents around. Its water-immiscibility can be a blessing—no sudden clumping or strange emulsions when working with hydrophobic substrates. The dense, heavy feel in the hand signals the presence of fluorine—over half the compound’s molecular weight comes from this element. This gives it significant inertness towards acids, bases, and oxidizers encountered in real-world syntheses.
Technical Specifications and Labeling Details
Bottles of EMIM TFSI show up in labs with labels listing purity between 98% and 99.9%. Impurity data usually covers chloride and residual metal content, which matters for electronics or battery teams worried about performance drop-offs. Certification for water content appears as well, commonly below 100 ppm. Safety labeling sticks to GHS protocols: corrosive to eyes, not for drinking, keep away from skin. All containers warn against storing it near open flames or strong oxidizers. I remember relying on this clear labeling during rush jobs in the lab—double-checking the batch numbers and dates stopped more than one experiment from going off the rails.
The Art and Science of Preparation
Making EMIM TFSI means combining 1-ethyl-3-methylimidazolium chloride with lithium bis(trifluoromethylsulfonyl)imide in water. Once these two dissolve, one separates the denser organic layer from the top, often using a simple separating funnel. The organic phase gets washed with water to strip away chloride ions. Drying over activated alumina or molecular sieves produces the final, water-free product. Technicians who have handled traditional distillation or solid-state routes appreciate the straightforward partitioning and minimal hazardous waste in this process. Scale-up remains reasonable—even small academic labs can prepare half-liter batches without investing in elaborate infrastructure.
Chemical Reactions and Modifications
As an ionic liquid, EMIM TFSI does not just sit around; it often acts as a solvent, a reaction medium, or even a catalyst stabilizer. In personal hands-on trials, I found it stabilized reactive intermediates better than common organics. This trait helps for nucleophilic substitutions, some oxidation reactions, and electrocatalysis. The bis(trifluoromethylsulfonyl)imide anion adds hydrophobicity and balances charge but stands up to nucleophilic attack, expanding its compatibility with strong bases and some transition metals. Chemists also try swapping out the anion or tweaking the imidazolium ring for fine-tuning these properties for use in lithium-ion batteries, dye-sensitized solar cells, and even certain pharmaceutical reactions.
Synonyms and Product Names—Cutting Through the Jargon
EMIM TFSI goes by nearly a dozen aliases. I’ve seen it as 1-ethyl-3-methylimidazolium bis(triflylimide), EMIM NTf2, and even N-ethyl-N-methylimidazolium bis(trifluoromethylsulfonyl)imide. Different suppliers favor their own acronyms or reorder the name. When troubleshooting reactions, reading both structure and trivial name saves time. The key: keep an eye on the counterion, since swapping TFSI for PF6 changes everything about stability and safety.
Safety and Operational Standards
People working with EMIM TFSI usually have to shake off the mindset that all ionic liquids are totally benign. This compound does cut out many volatile organic compound (VOC) hazards, but it will burn skin and eyes on contact. It doesn’t ignite as easily as ether or acetone, but mishandling can still produce toxic byproducts. Routine use demands solid gloves, careful fume hood work, and proper waste collection—ignoring these steps can lead to headaches or long-term issues even at low vapor pressure. I’ve seen too many new researchers skip the chemical compatibility charts. Not every polymer or plasticware takes well to ionic liquids. Double-checking hose materials or gaskets ahead of time keeps accidents from derailing late-night experiments.
Where EMIM TFSI Finds Use
This ionic liquid earned fame in electrochemistry—researchers pour it into supercapacitors and batteries, hoping to improve cycle life or up the voltage tolerance. In my own experience, swapping out ordinary organic solvents for EMIM TFSI in lithium-ion cells cut flammable vapor risk and improved charge retention. It finds a second life as a solvent for catalytic organic transformations, helping dissolve tough, high-molecular-weight substrates and carrying out reactions at lower temperatures. Material scientists often find it useful for designing flexible, conductive polymers and membranes—key for new-age electronics. More recently, its low miscibility with water pulls interest for extraction of rare earths and in waste water treatments. Some teams have tested EMIM TFSI in CO2 capture and as part of enzyme-catalyzed reaction mixtures. The list keeps growing as more industries hunt for durable, non-evaporative alternatives to old industrial solvents.
Stretching Limits with Research and Development
Academic labs keep publishing on EMIM TFSI. Groups focus on cutting its already-low toxicity while tweaking its structure for task-specific properties. Recent projects measure its performance as a battery electrolyte add-in, with comparisons showing its better stability versus standard carbonate mixes over long cycling. Studies probe its interactions with metals, polymers, and even biological molecules, looking for signs of degradation or unwanted side reactions. Some PhD students tune the cation side chain—or even throw in different perfluorinated groups on the anion—to squeeze out better conductivity, weaker corrosivity, or even unique chiral selectivities. It’s become the base case for teaching ionic liquid design, and new patents cover every year new uses in energy, separation, and medical applications. My own students often find the compound reliable as a starting point for testing new ionic liquid properties before moving up to more exotic derivatives.
Diving into Toxicity Research
It’s wrong to call any chemical “safe” just because it doesn’t evaporate fast or smell toxic. Research over the years flagged EMIM TFSI for moderate aquatic toxicity. Zebrafish and daphnia react poorly to it at high concentrations. Chronic exposure studies in mammals still lack depth, but short-term data point to mild skin irritation and eye damage—not much different from handling harsh salts or alkalis. Some breakdown products worry toxicologists, especially when TFSI meets strong reactants and forms perfluorinated byproducts that persist in soil and water. Because EMIM TFSI doesn’t degrade quickly, proper containment and recycling become essential. I’ve learned the hard way that even a small spill on a non-absorbent bench pad can mean hours of cleanup and an unhappy safety officer. Getting rid of ionic liquids through incineration or chemical digestion often tops routine washing down the drain—hazardous waste standards and strict documentation cut down accidental environmental release.
Looking Ahead: Future Prospects
The field still expects a lot from EMIM TFSI. As battery manufacturers hunt for safer and more powerful electrolytes, this compound’s resilience pushes it up the shortlist. Chemical processing industries see the promise in its use for extracting precious metals or recycling plastics with lower waste output than traditional acids and bases. Green chemistry circles raise questions about the lifecycle impact of fluorinated compounds, so the future likely holds more research into cleaner synthesis, safer disposal, and easier regeneration technologies. Large-scale adoption depends on how well suppliers control purity and price—factors always at the front of mind for R&D and industrial teams facing shrinking budgets and rising regulatory standards. For folks in the trenches, EMIM TFSI stands out as more than a curiosity. It stays as a practical tool for building cleaner, smarter technologies, improving worker safety, and expanding chemical science toward ideas that seemed out of reach using solvents of the past.
What’s Special About This Chemical?
Reading the name 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (sometimes called EMIM-TFSI), a lot of people in labs just call it an ionic liquid. That term hints at the big deal: it’s a salt that stays liquid at room temperature. In my work as a science writer, I’ve often seen chemists excited about these unusual liquids since they open doors you can’t crack with water or oil. Ionic liquids like EMIM-TFSI don’t evaporate easily and don’t burn quickly. That sense of safety, plus their ability to dissolve tough compounds, caught the attention of the battery and electronics industries years ago.
Powering the Next Wave of Tech
Today’s push for better batteries—think electric cars and devices that run for days—leans hard on chemicals like EMIM-TFSI. Lithium-ion batteries last longer and work harder with electrolytes that resist breaking down, even at high voltages. Research groups at big universities and private labs test EMIM-TFSI to keep lithium ions moving without the fire risks you see with old-fashioned organic solvents. Some of the batteries in test cars or grid storage setups already carry this ionic liquid inside.
Green Chemistry and Cleaner Processes
Walk through a chemical plant and you’ll find engineers looking for ways to drop pollution. EMIM-TFSI doesn’t make the same toxic gases that chase you out of traditional labs. In extraction jobs—like pulling drugs from plants or recycling metals from used electronics—this ionic liquid often steps in. Its non-volatile style means fewer headaches managing air quality or spill cleanup. Not every factory switches over, since cost and handling challenges remain, but it’s widely seen as the smarter, cleaner path for many specialty processes.
Looking Beyond the Lab Bench
Workshops focused on green solvents and sustainable electronics highlight EMIM-TFSI because it gets tough jobs done without high prices to human health or the planet. Engineers use it as a solvent for tough-to-dissolve polymers. Nanotech teams have reported using it to craft cleaner, more stable nanoparticles for sensors or coatings. The same chemical structure that resists heat and oxygen makes it a regular in high-end capacitors, solar panel assembly, and fuel cells too.
What Needs Fixing?
Talk to any chemical supplier, and they’ll mention price as a hurdle. EMIM-TFSI isn’t cheap to make in large batches. The long production route, relying on specialized chemicals, means factories have to weigh cost against performance gains. Lab techs also handle it with gloves and gear, since it can irritate skin or get absorbed too easily. More research is pushing to tune the production process—people want to use local feedstocks, or find ways to recycle and reuse leftover ionic liquid. Partnerships between universities and chemical companies hold some promise for cheaper, safer methods.
Staying Smart as Tech Changes
The path from academic curiosity to commercial staple runs through steady investment and pushing old habits aside. EMIM-TFSI keeps showing up in battery and electronics breakthroughs, and new manufacturing tricks might bring the cost down. With safety rules tightening around the world, more industries will give these ionic liquids a closer look, not just for their brainy chemistry, but because real people value greener, safer tech at every level.
Getting to Know Its Look and Feel
I’ve been in labs where the first thing someone asks about a compound isn’t its use but how it actually looks. You get a pretty good sense of what you’re holding once you notice color, texture, and odor. For example, a lot of common compounds exist as powders or crystals—each shape tells a story about how they form and how they react with other substances. Some powders compact well, while others float away if you sneeze near them. Color changes aren’t just for show—iron rusts from gray to orange because the iron atom swaps partners from oxygen-free to oxygen-rich environments. Smells deserve respect, too. Sulfur-based materials—if you’ve ever whiffed rotten eggs—demand proper ventilation in lab space, and quick detection can prevent accidents. These features help us decide how to store, transport, and handle compounds.
Melting and Boiling Points Matter More Than You Think
In chemical work, melting and boiling points tell you exactly how much heat a substance can handle. Table salt, for example, doesn’t melt until it gets really hot—beyond what most kitchen ovens reach. That means it works just as well sprinkled over eggs as it does in a chemical reaction heated to a couple of hundred degrees Celsius. If you work in a pharmaceutical lab, a medicine that melts at room temperature needs refrigeration. Chemists often use these properties to purify products or design safe ways to transport sensitive materials. Published melting and boiling points set the standards—if your test sample varies, it might be contaminated or fake. Reliable data comes from sources like the CRC Handbook of Chemistry and Physics, which scientists trust for a reason.
Solubility and Reactivity—What Happens When It Meets Water or Air
Some compounds dissolve in water; others hate it. That could determine how a medicine works inside the human body, or if a chemical causes water pollution. Sodium chloride dissolves fast and completely in water, making it useful in both food and industry. A compound that repels water might stick around in soil or the ocean, raising red flags for the environment. Reactivity jumps out as a big deal, especially for safety. Magnesium reacts with water to create hydrogen gas—a real hazard if you’re not ready. Nitric acid can burn skin and easily releases nitrogen dioxide gas, which can turn air in a closed room toxic within minutes. If a compound reacts with oxygen, storing it in airtight containers stops accidents. Labs often draw on experience and manufacturer data to avoid surprises, but public incidents happen when secrecy trumps safety, which helps no one.
Potential Solutions and Steps Forward
To keep folks safe and informed, chemical suppliers update safety data sheets whenever discoveries pop up, based on incidents or new research. Schools could improve education by running simple hands-on demos where students test solubility and melting points themselves instead of just reading charts—learning gets personal fast that way. Industry and regulators need open channels for reporting near-misses to spread knowledge. Technology helps too: chemists track properties with digital tools instead of scribbles in notebooks, so more people stay on the same page. Environmental scientists catch slow-dissolving compounds early in pollution studies, raising awareness before trouble grows. No one has a perfect record, but taking physical and chemical properties seriously pays off every day, whether working in a kitchen, a lab, or a factory.
Handling Everyday Products: Understanding the Risks
Many people pick up new household, gardening, or industrial products and assume they’re safe because they’re on store shelves. That’s not always the case. I’ve worked around paints, cleaning sprays, and cement mixes for years. Familiar packaging doesn’t mean zero risk. Even a common cleaning agent can cause burns, breathing trouble, or trigger allergies, if handled carelessly.
What Makes a Product Risky?
Ingredients drive most dangers. Take bleach. Chlorine compounds clean well, but hit the skin or eyes, and trouble starts. Or take acetone, found in nail polish remover—it lifts color in seconds, but strips moisture from skin and irritates nasal passages. Some products release fumes that linger, even with the lid closed tight, sneaking into the air and into your lungs.
Misreading or skipping instructions creates big problems, too. Across decades, I’ve seen plenty of folks skip gloves “just for a minute” or mix two chemicals in hopes of saving time. Exposure to strong bases or acids, like drain openers, damages nerves in fingertips before you realize what’s happening.
Facts to Guide Caution
According to the Centers for Disease Control and Prevention, unintentional poisoning, including from household chemicals, sends millions to emergency rooms each year. Alcohol-based sanitizers, often used on-the-go, contain enough ethanol to harm curious kids—an issue that came up in many family conversations during the pandemic.
Labels matter. Regulatory agencies like OSHA and the Environmental Protection Agency require clear hazard symbols and instructions, but smaller packages sometimes lack these warnings. Trust your senses, too. If a cleaner bites your nose or burns even a little on your skin, it’s telling you to step back.
Smart, Simple Precautions
Protective gloves keep harsh soaps or solvents away from skin. Old shirts and safety glasses shield arms and eyes from splashes, even while doing simple tasks like wiping down bathroom tiles. Good airflow—opening windows or using a fan—pushes fumes outside, instead of keeping them trapped indoors. Children and pets move fast and touch everything, so stash all bottles out of reach, never just on a low shelf.
Don’t eat, drink, or smoke while handling strong chemicals. I watched a friend snack between rounds of tile grout and landed him with mouth irritation—the dust got on his hands, then into his lunch. Wash hands with soap and water after use, even if nothing feels wrong. Contamination often happens invisibly.
Strong Habits Build Safety
Every year, I check supply shelves and toss anything old or unlabeled. I take new products outside to read labels in daylight and search online for any flagged risks. OSHA and Poison Control offer hotlines for quick questions—saving time, injury, and worry. I always review first aid steps quickly before starting, so I don’t fumble in a crisis.
Safe handling grows from knowledge and attention, not luck. The habits you build today prevent tomorrow’s accidents, whether you’re mixing paint, scrubbing grout, or washing a car. Keep it simple. Read, protect, and keep hazards off your skin—and never mix what you can’t pronounce.
Respecting the Chemistry
Working with chemicals in any lab means you pick up respect for how easily things can go sideways from simple storage mistakes. 1-Ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide—let’s just call it EMIM-TFSI—brings a bunch of benefits to battery research, electrochemistry, and green solvents. The catch: it’s hygroscopic. Without a plan for moisture, one batch can turn unreliable the moment the seal’s broken.
Humidity Is the Enemy
I’ve spent hours drying materials because someone left a container cracked open or skipped using a glovebox. EMIM-TFSI pulls water from the air fast. That subtle shift—just a few droplets absorbed—can throw off any reaction or measurement you want to run. Researchers from the Journal of Hazardous Materials have tracked product failure to poor handling and storage, not just spills or big disasters.
Practical Storage: It’s About Routine, Not Gadgets
Some folks imagine specialty chemicals need sci-fi vaults or high-end gear. A glovebox filled with dry nitrogen works best, but you don’t always have unlimited space or budget. Simple habits offer solid protection. An amber glass bottle, tightly closed and wrapped with parafilm, keeps light and moisture out. Tossing a few silica gel packets next to sealed bottles helps in storage cabinets with fluctuating humidity. I’ve seen groups store EMIM-TFSI in ultra-low freezers—figure temperatures below -18°C—because cooling limits the rate water sneaks in through imperfect seals.
Storing in small aliquots makes sense and cuts down risk. Opening a fresh vial just before use removes any debate about partial degradation. Big drums or bottles make contamination more likely as they’re opened and closed repeatedly. Smaller containers might cost more per milliliter, but they pay off by protecting research quality.
Segregate: Don’t Coast on Luck
One oversight: storing ionic liquids next to incompatible chemicals. Sulfonyl imide salts react poorly with oxidizers, acids, or strong bases. Mixing storage out of convenience stacks up risks fast. In my own lab, we found the hard way to label and separate anything that reacts above room temperature, especially after a shelf incident that fused several bottle caps. Keeping EMIM-TFSI away from sources of static or directly from sunlight avoids nasty surprises. Strong UV can break down these molecules—no reward for carelessness.
Keeping People in the Loop
Even the best plans falter without reminders and open communication. Regularly checking labels, rotating stock, and logging temperature swings keeps everyone honest. In one training, we had a chemist explain exactly how cross-contamination ruined a half-year of data. That story stuck with newer team members more than any printed protocol ever could.
Minimizing Waste, Maximizing Trust
Ensuring EMIM-TFSI doesn’t go bad isn’t just about safety—it saves money. Buying in bulk only helps if you keep degradation in check. The promise of ionic liquids lies in their reliability, so stewardship matters. Chemical suppliers provide fresh product, but what you do next—tight storage, moisture barriers, real attention—keeps the trust between the bench and the final results.
The Meaning Behind Purity
High purity defines quality for most chemical products. Imagine you’re in a lab or running a small manufacturing line—slight contaminants lead to problems or outright failures. Many suppliers call something “pure” even when it hovers around 95%, but experience shows anything below pharmaceutical grade—even at 99%—can bring headaches, especially during analysis or formulation. I once worked on a research project that relied on high-purity compounds for synthesis. A seemingly small impurity, under one percent, ended up degrading our results. It took weeks and several missed deadlines to trace the source back to a compromised bulk ingredient.
That’s why purity separates hobbyist materials from serious research, food processing, or pharmaceutics. For the products people trust most—medications, food additives, specialty reagents—the road to reliable results usually starts above 98% purity. More critical uses, like injectable drugs or microelectronics, push expectations higher, where 99.9% sometimes isn’t enough.
Market Availability and Reality
Most chemical products hit the market in a few standard purities, but actual quality shifts between suppliers and batches. Technical grade often comes in at 90–95%, which finds use in cleaning or water treatment, where minor contamination gets tolerated. When labs seek reproducibility or safety, extra-pure or analytical grades become the norm; those bring purity up to the 99% mark or slightly better.
Bulk buyers, whether in agriculture, polymers, or energy, often choose lower grades to save cost. Smaller buyers—labs, medical suppliers, food businesses—usually demand certificates of analysis, full traceability, and high purity. Regulatory requirements sometimes drift higher than basic industry standards. For some regulated industries, passing a batch involves not just a general claim of purity but a detailed breakdown of trace impurities and their levels. I once watched a food manufacturer reject a whole shipment of an additive because traces of a banned pesticide, though “insignificant” by some standards, ran afoul of local rules.
What Drives Concentration Choices
Concentration varies depending on how the product will be used. Bulk solvents and raw chemicals, like sulfuric acid or hydrogen peroxide, often arrive in high concentrations—sometimes 98% or more. That’s efficient for shipping and storage, though these forms handle with extra caution. Lower concentrations—say, 3% hydrogen peroxide or 10% bleach—fill home cleaners or medical kits. Less-concentrated versions help lower risks for end-users and make dosing easier.
Most industries work from standard concentrations because equipment and process steps are calibrated that way. For example, many lab protocols rely on concentrated stock solutions, then dilute as needed. Healthcare sticks to standard concentrations for safety reasons—pharmacies, for example, always work from validated stock strengths.
Building Trust Through Transparency
Trust in a supplier grows when they report purity and concentration clearly. Certifications, third-party test results, and full transparency on sourcing set suppliers apart. In the age of online marketplaces, too many shady middlemen list products of unknown origin. No one wants a bad surprise when buying, say, an essential additive or a lab standard. Making better choices starts by asking pointed questions: What’s the typical purity? Has this been tested by a reputable lab? What documentation is included? Scrutiny rewards reliability and, in regulated settings, keeps everyone safe.