1-Ethyl-3-Methylimidazolium Dicyanamide: A Deep Dive
Historical Development
1-Ethyl-3-methylimidazolium dicyanamide entered the spotlight at the dawn of the 21st century, back when the world of ionic liquids took off as chemists chased alternatives to toxic solvents and outdated reaction media. In those early years, scientists spotted the imidazolium core as a promising platform for building stable, tunable liquids. Suddenly, this ionic liquid turned up on lab benches from Berlin to Tokyo. Its rise isn't about hype—it’s about utility. Solvent systems that don't evaporate easily, conduct electricity, and shrug off moisture started changing the way materials scientists, engineers, and green chemists did their jobs. The story of this compound runs parallel to the whole movement toward greener chemistry, giving those in research a tool that skipped the volatility and flammability of older solvents.
Product Overview
Today, the clear-to-yellowish liquid listed on chemical suppliers' catalogs as 1-ethyl-3-methylimidazolium dicyanamide finds its way into a wide variety of labs. Easy to handle at room temperature, it attracts attention for its unusual combination of low viscosity, near-zero vapor pressure, and high ionic conductivity. Unlike traditional solvents, it doesn't vanish into the air or catch fire from a random spark, so the people who work with it worry less about inhalation or explosive hazards. This compound appealed to many for making processes more sustainable—one container might get reused across lots of test runs without losing its punch.
Physical & Chemical Properties
On the bench, this ionic liquid flows clear, looking somewhat like a light oil. It resists forming crystals unless chilled below -21°C, staying liquid just by sitting on the shelf. The density falls between 1.05–1.1 g/cm³, and it doesn't mix in with water quite as freely as some other ionic liquids, though it dissolves many organics. Another thing I noticed: it carries a faint, bitter odor and a slippery feel. Chemically, the dicyanamide anion offers good stability. It won't decompose below 200°C, and it doesn’t interact much with acids or neutral salts, so mixing in other reactants is pretty safe. The imidazolium cation acts as the polar anchor, resisting both oxidation and reduction up to a decent voltage window, which explains why energy device engineers like to use it in batteries and capacitors.
Technical Specifications & Labeling
Quality-wise, suppliers test for water content—usually holding below 0.2%—to guarantee reliability for electrochemistry. Purity hits at least 98% by HPLC or NMR. Transporting this material calls for clear GHS labeling, reflecting its moderate toxicity. Most bottles ship in dark glass or HDPE to avoid UV breakdown, with hazard symbols in plain sight. Labels must read out '1-ethyl-3-methylimidazolium dicyanamide' alongside standardized product codes and safety phrases (like H302/H312 for harmful if swallowed or in contact with skin). Every reputable supplier provides detailed safety data sheets, supporting safe routine work and helping buyers understand how trace impurities could hurt processes.
Preparation Method
My time in the lab has shown that the simplest route to this compound starts with stirring 1-ethyl-3-methylimidazolium chloride in water or acetonitrile, then slowly adding sodium dicyanamide. The resulting salt exchange causes sodium chloride to drop out while the ionic liquid stays dissolved. Filtration, followed by careful evaporation under vacuum, produces a pure, clear liquid. Good glassware and patience mean fewer headaches later—any trace sodium chloride left behind could throw off conductivity in electronic applications. Some larger plants run this synthesis continuously, swapping glass flasks for stirred reactors and built-in dryers, but the steps remain the same: salt metathesis, filtration, solvent removal, and drying under reduced pressure.
Chemical Reactions & Modifications
This liquid isn’t just a bystander in the flask—it can take part in chemistry. While the imidazolium backbone stays inert under most conditions, dicyanamide brings interesting possibilities. Many researchers use it as a solvent for metal ions, finding that it stabilizes metals in solution far better than water or alcohol. In some cases, the dicyanamide ion acts as a tiny ligand, wrapping around transition metals and changing their catalytic skills. Synthetically, it can foster C–C bond formation or swap with halide anions in situ, which turns this compound into a gateway material for more complicated ionic liquids. Modification follows from simple salt exchanges, but some groups have explored grafting the imidazolium ring onto solid surfaces, making supported ionic liquid catalysts that can be reused again and again—a boon for sustainable chemistry.
Synonyms & Product Names
Users in the industry might know this liquid by shorthand as [EMIM][DCA], EMIM-DCA, or simply EMIM dicyanamide. The IUPAC nomenclature makes it sound complicated—“1-ethyl-3-methylimidazolium dicyanamide”—yet most researchers stick to the abbreviations. When searching catalogs, the CAS number 258716-79-9 helps filter out similar but distinct imidazolium salts. A few suppliers use trade names, but for reliability in research, the field’s stuck close to the core formula and acronym.
Safety & Operational Standards
Even green credentials can't erase all risks. 1-Ethyl-3-methylimidazolium dicyanamide absorbs through the skin and can cause irritation or more serious problems with repeated exposure. Proper lab work means donning nitrile gloves, checking for good chemical hoods, and making sure bottles stay sealed. Spills clean up easily with absorbent, though floors get slick fast. Disposal follows local hazardous waste protocols—pouring it down the drain doesn’t cut it, since dicyanamide’s persistence in water could disrupt biological systems. Many companies keep logs for ionic liquid use, tracking volume and exposure. Training new chemists on these standards builds habits that cut long-term accidents. I’ve seen labs without robust safety practices run into trouble, not from dramatic fires but from gradual solvent absorption and chronic exposure, which snuck up on unsuspecting workers.
Application Area
This compound doesn’t stay stuck in academic glassware. Large-scale lithium battery makers appreciate its role as a conducting salt, pushing up performance and lifespan while reducing fire risk. Analytical chemists pick it for dissolving stubborn organic or polymer samples, sometimes detecting trace analytes that water or methanol miss. In catalysis, [EMIM][DCA] serves as a reusable medium, keeping expensive metals in solution for longer runs, reducing both cost and waste. Some groups push its use in CO2 capture, with the liquid absorbing carbon dioxide much more efficiently than amines. Its low volatility means environmental engineers can design processes without constant solvent loss, which fits well with sustainable design philosophies. In my own work, swapping traditional solvents for this ionic liquid unclogged several sluggish reactions, revealing cleaner products and less chemical byproduct.
Research & Development
The push for greener, smarter chemistry keeps labs tweaking this ionic liquid’s structure. Research groups blend dicyanamide with other imidazolium variants, tuning their melting points and conductivity for specific tasks. There’s broad investigation into using these mixtures as electrolytes not only for lithium but for magnesium and sodium batteries—future energy storage hinges on these advances. Some development teams experiment with immobilized forms, tethering the cation to solid supports for easier separation and recycling. Analysis of ion mobility, viscosity under pressure, and breakdown at high voltages feeds directly into better fuel cells and sensors. Anyone watching conference proceedings in green chemistry sees a steady stream of posters and talks about fresh ways to deploy and improve this liquid, powered in large part by open collaboration between university and industry partners.
Toxicity Research
For all its appeal, responsible use means understanding its dark side. Recent animal studies and aquatic toxicity screens suggest dicyanamide-based ionic liquids break down slowly in the environment and can disrupt cell growth at elevated doses. Chronic exposure has been linked to enzyme inhibition and, in rare cases, mild neurotoxicity. Lab workers run less risk than industrial operators, but every safety summary reminds us to limit contact, avoid ingestion, and ensure spills never reach open drains. Waste treatment plants often lack tools to process these ionic liquids effectively, so segregated disposal is now standard at major research institutions. These real-world toxicity profiles help set occupational exposure limits and drive the search for even safer next-generation solvents that keep the strengths but lose the lingering health questions.
Future Prospects
What lies ahead for 1-ethyl-3-methylimidazolium dicyanamide? Its place in battery innovation looks solid. As interest grows in magnesium and sodium chemistries, this ionic liquid’s tuneable anion keeps finding matches that boost conductivity and long-term cycle stability. In green manufacturing, the pressure to cut emissions and solvent waste has more producers swapping old-school solvents for this ionic alternative, especially as REACH and similar regulations get stricter. Interdisciplinary teams are now pairing ionic liquids with membranes, catalysts, and nanomaterials to squeeze out even more value. If new routes can address toxicity and drive costs down, the next decade might see widespread adoption in everything from power grids to pharmaceutical synthesis. Knowing how far this compound’s already traveled—from a niche curiosity to a tool shaping entire industries—I see it occupying a lasting spot in modern chemistry’s toolbox.
Getting Practical with Modern Chemistry
Ask anyone who’s ever worked in a chemical lab about ionic liquids. These aren’t just fancy salt solutions; they’re some of the most useful tools developed over the last couple decades. Among them, 1-ethyl-3-methylimidazolium dicyanamide shows up again and again in research papers, industrial catalogs, and more importantly, in projects that stretch well beyond the bench. My time running tests with advanced solvents taught me real innovation comes from what actually solves problems on the ground. This ionic liquid fits that bill and deserves some attention for what it brings to multiple fields.
Making Chemistry Cleaner and Faster
Most people never think about where cleaner, more sustainable chemical reactions start. Scientists working on green chemistry are hungry for smarter solvents, and this unique ionic liquid stands out. Instead of sticking with old-school solvents fraught with safety headaches, labs have turned to 1-ethyl-3-methylimidazolium dicyanamide for reactions like alkylation, hydrogenation, and even enzyme-catalyzed processes. Anyone tired of inhaling harsh fumes or battling fire hazards will appreciate something that runs under mild temperatures and helps avoid toxic byproducts. This cuts waste, protects the lab crew, and still gives high yields.
Research from the early 2010s made clear just how much of a game-changer this liquid proved in these reactions. For example, in cellulose processing, it breaks down raw plant matter more efficiently, helping produce biofuels or specialty chemicals. Industry loves lowering energy bills and handling less sludge.
Electrochemistry: Batteries Get a Boost
Battery makers face a big headache—keeping devices reliable without sacrificing safety or lifespan. After working on battery electrolytes, I saw firsthand how flammable mixtures spark fires and spook safety teams. Dropping this ionic liquid into lithium-ion battery research brought a non-volatile, thermally stable option that actually holds up under tough cycling tests. Researchers have published findings on its performance as both the main solvent and as an additive that helps slow down capacity fade. As electric vehicles and backup grids expand, the hunt for safer battery chemistry isn’t slowing down. This material keeps showing real promise.
Cleaning Up Metals and Materials
Industrial metal plating and electrorefining always leave a mess behind, from toxic rinse water to spent solutions full of heavy metals. 1-ethyl-3-methylimidazolium dicyanamide offers a much less polluting alternative for selectively dissolving or separating metals like silver, gold, and copper. I’ve seen small shops improve recovery rates by using this type of liquid during extraction, often burning through fewer hazardous chemicals in the process. Even precious metal recycling—like salvaging gold from scrap electronics—gets easier with this liquid handling the job.
A Tool That Blends In
One thing that sticks out: this compound slips into different roles without skipping a beat. In some labs, it’s dissolving polymers so researchers can spin out designer fibers for electronics or filtration. In others, it’s taming corrosion or helping pharmaceutical teams purify final products. Its low volatility, strong solvating power, and chemical stability are more than just selling points. These qualities shave time and headaches from real projects.
Moving science forward often means finding a tool that does more with less harm. 1-ethyl-3-methylimidazolium dicyanamide quietly proves itself in these diverse roles. Engineers and chemists are finding smart ways to use it, blending practical chemistry with a much-needed push for safer, cleaner manufacturing and energy.
Understanding What You're Working With
Anyone stepping into a lab knows that chemicals demand respect, and 1-Ethyl-3-methylimidazolium dicyanamide isn’t an exception. Its name alone raises questions. Used in advanced batteries and heat-transfer research, this ionic liquid stands out for its low volatility and conductivity. Despite sounding exotic, it comes with down-to-earth rules. I've worked around similar organic salts for years; they catch you off guard if you get careless. The greatest safety tool is not a fancy fume hood — it’s good habits and staying alert.
Direct Contact: A Shortcut to Trouble
Some folks make the mistake of brushing off gloves or skipping goggles since it doesn't fume like strong acids or bases. Big mistake. Even liquids considered "low hazard" can irritate your skin or eyes, and a splash in the wrong place puts you in the emergency room. Nitrile gloves, long sleeves, and eye protection work for almost anything but especially for dicyanamide compounds. I found that even a bit of this stuff which got on my skin left unmistakable dryness and mild irritation. Washing up right after handling becomes second nature fast.
Ventilation Is Your Friend
Being odorless doesn’t mean safe to breathe. During my time handling ionic liquids, even the best-behaved ones occasionally surprised me with allergic reactions in sensitive co-workers. Always use it under a fume hood or in a well-ventilated lab. It’s those little droplets or fine splashes that you don’t notice that end up in the air or on surfaces. Clean up immediately, no exceptions. Respirators stay in reserve for larger spills, but I always check the air flow before opening any bottle.
Spill Response: No Drama, Just Fast Action
I’ve seen people freeze during a spill, thinking they need hazmat suits for everything. A small spill just needs patience and the right absorbent. Blot, scoop, and collect it in a labeled waste container. Don’t hose it down the sink; dicyanamide ions break down slowly and cause headaches for water treatment plants. My old lab had one clear rule: never walk away from a spill, even "just a little" one. Finish the job, then wash the area with soap and water.
Sensible Storage: Out of Reach, Out of Mind
This chemical remains stable for months, but that’s no excuse to stash it next to your lunch or coffee. Use tightly sealed containers and assign a spot in the chemical cabinet away from strong oxidizers, acids, or base stocks. Humidity slowly degrades many ionic liquids; so I never leave these bottles open longer than necessary. If sharing a space with new students, clear labels and written instructions keep everyone from guessing what’s safe to touch. I always include a handwritten note about the main hazards to jog people's memory.
Respect for the Clean-Up Crew
Waste disposal gets overlooked, but I learned the hard way not to treat “universal waste” as a catch-all. Dicyanamide salts go into sealed, labeled cans. Casual pouring into organic waste means someone downstream faces added risk. A few minutes sorting and double-checking containers beats hours untangling a mess after a leak or mixing incident. My coworkers appreciated when we all followed the same map for disposal; it cut down on cross-contamination and drama.
Training Beats Overconfidence
Guidelines aren't just paperwork. They save skin and, sometimes, careers. I always recommend newcomers read the chemical safety data sheet at least once, no exceptions, and share close-call stories without blame. No one is immune to mistakes, but teams that talk honestly about them stay safer. If you’re not sure about a procedure, ask. The risks stack up fast, and keeping things simple is smarter than gambling with your health.
Understanding Its Structure and Formula
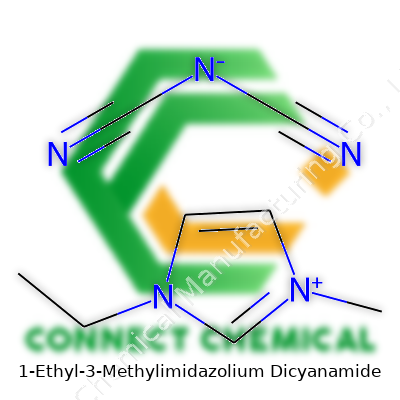
1-Ethyl-3-methylimidazolium dicyanamide shows up in labs and research papers under the formula C8H11N5. Friends in the field call it [EMIM][DCA]. That shorthand points to its split personality: the cation, 1-ethyl-3-methylimidazolium, and the anion, dicyanamide. Picture a molecule not shaped by rigid rules or old chemistry traditions, but by clever thinking in ionic liquids research.
Imidazolium rings resemble a five-membered ring with two nitrogen atoms at positions 1 and 3. Add an ethyl group to one nitrogen and a methyl to the other; it’s a recipe for a liquid that does not easily fit into the familiar categories of solvents and salts. The dicyanamide anion, with its two cyanide (−C≡N) groups attached to a single nitrogen, carries the formula N(CN)2−. Each piece is stable and together, they form a salt that hardly ever crystallizes but flows at room temperature.
Why Structure Matters in the Real World
A closer look at those five-membered rings and the arrangement of carbon and nitrogen atoms explains why labs across the world keep exploring ionic liquids like [EMIM][DCA]. An imidazolium backbone grants remarkable stability, surviving high temperatures and staying liquid even when much of the lab gets chilly. This matters more than ever as green chemistry edges out old, toxic solvents. Researchers keep searching for smarter, cleaner ways to dissolve, mix, and separate chemicals, and [EMIM][DCA] keeps stepping up.
Dicyanamide as an anion helps lower viscosity and offers unique coordination chemistry, which makes this compound valuable in catalysis and electrochemistry. Standard organic solvents struggle to match ionic liquids like this in performance and versatility. The chemical’s low volatility cuts back on air pollution and workplace hazards. Unlike volatile organic compounds, [EMIM][DCA] doesn’t evaporate everywhere, so spill risks and toxic exposures drop way down. Safety and environmental records benefit from choices like this.
Real-Life Challenges and Looking Ahead
Despite the advantages, 1-ethyl-3-methylimidazolium dicyanamide carries baggage. Some studies point out that dicyanamide-based ionic liquids break down in the environment and may not biodegrade quickly. Concerns about toxicity to aquatic life and the unknowns of long-term impacts land this compound under the watchful eye of regulatory agencies. The pressure mounts: society wants innovation, but also responsibility. Academic journals and industry experts, including the American Chemical Society, have raised red flags where safety data run thin.
Researchers continue to investigate ways to tweak the structure—changing the side groups on the imidazolium ring, or even switching the anion entirely—to tackle stability and toxicity issues. Promising directions come from tailoring new ionic liquids with biodegradable or recyclable components. Collaboration with toxicologists and environmental scientists helps steer progress toward solutions that balance performance with stewardship.
Trust and Chemistry’s Next Steps
As the world keeps searching for alternatives to old chemical standards, [EMIM][DCA] and compounds like it serve as stepping stones. Their unique structures open doors for safer and cleaner lab work. By being open about structural details and potential problems, chemists build trust—one molecule at a time. Working together, the field can keep progress moving without sacrificing safety or the planet.
Why Storage Matters for 1-Ethyl-3-Methylimidazolium Dicyanamide
People working in research labs and advanced manufacturing often meet 1-Ethyl-3-Methylimidazolium Dicyanamide—sometimes called an “ionic liquid” in technical circles. This stuff helps drive innovation in green chemistry, battery improvements, and separation processes. But it’s not a compound to treat lightly. Bad storage habits cause spills, trigger weird chemical reactions, or hit your safety record hard. Years spent around solvents and specialty chemicals taught me one thing: even curious new liquids earn the same respect as seasoned hazards.
Temperature and Humidity Counting Against You
Set up your storage area in a cool, dry section of the lab. Forget windowsills and benches near radiators. Ionic liquids have quirks: some—like this one—pick up water from the air. Once the water gets in, those chemical properties change. Reactions go sideways or tests fail, nobody wants to repeat a whole experiment for such a basic mistake. Use refrigeration to slow down the uptake of moisture and keep degradation at bay. Aim for temperatures below 25°C if you can, and lower with a monitored fridge for long sits. Silica gel packets in your storage cabinet offer extra protection against humidity.
Choose the Right Container
Glass bottles work best. I’ve watched plastics soften or turn stained with ionic liquids over a few weeks. High-purity glass avoids funny interactions and keeps your samples from leaching substances. Never settle for a loosely closed lid. Screw caps with PTFE liners keep both air and stray moisture out. Watch for cracked threads—one missed inspection and you might walk in on an ugly puddle.
Avoid Direct Light and Heat
Direct sunlight changes the equation completely. UV rays degrade chemicals, sometimes turning safe liquids into something that gives off toxic byproducts or colors your results the wrong way. Direct heat does the same. Blacked-out or amber bottles inside shaded cabinets work best. My first boss drilled that into me; I had to cover labels entirely, relying on coded tags only I understood.
Safety Labeling and Good Record-Keeping
Even in fast-moving labs, labeling every bottle with the chemical’s full name, date of receipt, and hazards keeps headaches out of your life. Too many times, I’ve watched confusion spread because somebody refilled an old solvent bottle “to save time”—and found themselves rewriting a whole logbook. Tag 1-Ethyl-3-Methylimidazolium Dicyanamide as toxic and harmful to aquatic environments, because local rules and global guidelines expect it.
Separation From Incompatible Materials
The bottle shouldn’t share a neighborhood with acids, oxidizers, or materials prone to hydrolysis and corrosion. Even in a packed cupboard, take the time to keep like with like. Shelves marked for ionic liquids only—or a dedicated fridge—shields everyone involved. Cross-contamination, which I once saw trigger a nasty cleanup and waste disposal run, always costs more than planned separation up front.
Emergency Planning
Store spills kits and absorbents nearby, not down the hallway. Quick response keeps you safe and the chemical out of drains or environmental waste problems. Clear procedures for cleanup, disposal, and reporting mean less confusion if something goes sideways. Sharing these habits with new researchers sets good habits early.
Final Thoughts
Safe storage of 1-Ethyl-3-Methylimidazolium Dicyanamide means less risk, fewer ruined experiments, and stronger compliance in the eyes of regulators. Chemistry always rewards respect and preparation. Set your standards high, and both results and safety will follow.
Digging Into the Risks
Spotting a chemical name like 1-ethyl-3-methylimidazolium dicyanamide in lab supply catalogs usually means you’re diving into the world of ionic liquids. Companies and university researchers prize these liquid salts for their ability to dissolve just about anything and remain stable under tough conditions. They help clean up industrial reactions and offer greener alternatives to traditional solvents. But while promoters often pitch ionic liquids as safe for the planet, the reality looks more complicated.
Messy Truths About Exposure and Health
No one likes to imagine a lab accident, but spills and splashes aren’t rare in real research or busy production plants. I once watched a bottle tip over in a student’s glove, soaking her palm. The label looked menacing, with warnings about cyanides and potential skin irritation. Not all safety data paint a clear picture for 1-ethyl-3-methylimidazolium dicyanamide, yet animal studies raise concerns. Rats exposed to dicyanamide-based ionic liquids displayed liver and kidney stress, which doesn’t get brushed off lightly.
Skin and eye irritation come up in safety profiles, and the substance’s cyanamide group rings alarm bells for toxicologists. Swallowing enough of it can trigger harmful reactions that go far deeper than temporary discomfort. Still, gaps remain in the data, especially on long-term effects or reproductive impacts in humans. Regulators and manufacturers often fall behind the curve; they don’t always publish comprehensive results for new industrial chemicals.
Environmental Footprint Looks Bigger Than Expected
Ionic liquids built their “green” reputation by replacing flammable, volatile solvents in research. Dicyanamide-based versions don’t release clouds of chemicals into the air, but they do not vanish into thin air either. University projects tracking their breakdown in rivers discovered that these chemicals don’t disappear quickly. Small aquatic organisms, like daphnia, struggled to survive as trace levels built up in lab aquariums. In one EPA report, dicyanamide ions scored moderate on aquatic toxicity tests and resisted full breakdown under sunlight or bacterial digestion.
So while nobody expects barrels of this chemical to pour into rivers, even modest leaks add up in manufacturing hubs. Once in water, ionic liquids can linger, and nature doesn’t break them down overnight.
Room for Safer Practices
Every tool in chemistry carries tradeoffs. People working in research or manufacturing settings hold the first line of defense through habits like gloves and careful storage. But personal protection isn’t enough on its own. Chemical manufacturers should fund deeper studies, especially focusing on how these substances behave in real-world ecosystems and how they interact with living tissues over years, not just days.
Green claims deserve skepticism unless the product lifecycle, from synthetic route to disposal, gets a real audit. Companies ought to design ionic liquids that break down more easily, or at least provide safe waste management options. Big universities and industry groups could partner for open chemical safety data, giving everyone a clearer picture of risk.
The idea that a label reading “low volatility” always means “non-hazardous” has let too many chemicals slip past scrutiny. Honest conversation about tradeoffs and regular reviews of emerging data will guide safer choices over time.