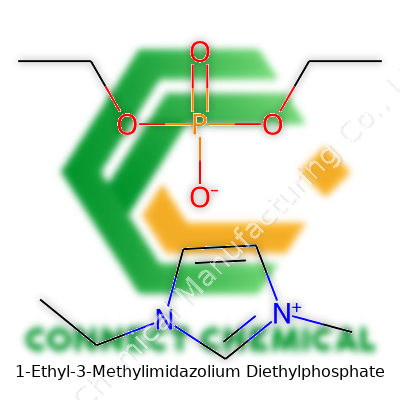
1-Ethyl-3-Methylimidazolium Diethylphosphate: A Deep Dive
Historical Development
1-Ethyl-3-methylimidazolium diethylphosphate belongs to a class of chemicals known as ionic liquids, which started gaining attention in the late twentieth century. Scientists began searching for solvents that could replace volatile organic compounds after tightening environmental regulations and increased awareness of occupational hazards. Early research into ionic liquids exploded during the 1990s, when researchers noticed how the structure of these salts produced unusual properties, such as low volatility, high thermal stability and the potential for “green” chemistry applications. By the end of the ‘90s, this material—often abbreviated as [EMIM][DEP]—had become one of the most recognized ionic liquids available to labs and the first companies that saw value in these modern solvents. As industrial players pushed for safer and more efficient processes, [EMIM][DEP] entered the scene as a promising candidate for various new applications. I remember the surprise among chemists who saw how these substances could carry out the role of solvents, but not release sharp odors or pose the same risk of flammability as familiar lab standards like acetone or toluene.
Product Overview
1-Ethyl-3-methylimidazolium diethylphosphate comes as a pale, often colorless or slight yellowish liquid, and it stays liquid at room temperature. Its formula blends an imidazolium cation with a diethylphosphate anion. The resulting product stands out for dissolving both organic and inorganic materials, and it manages this without producing harsh fumes. Sources show [EMIM][DEP] typically comes packed in sealed brown bottles or metal drums, protected from light and moisture. Lab workers favor it for its low odor, while manufacturers point to its practical, liquid state at ambient temperatures as an advantage for handling and mixing.
Physical & Chemical Properties
Under standard conditions, [EMIM][DEP] displays a melting point below -20°C, so it rarely crystallizes during routine storage. Its density hovers near 1.15 g/cm³ at 25°C. The liquid resists decomposition up to roughly 240°C. It responds poorly to water, rapidly dissolving but without precipitating out, and presents a strong ionic character without introducing electrical conductivity on par with salts dissolved in water. The phosphate group gives this compound a mild hydrophilicity, opening the door to aqueous-phase chemistry, while the imidazolium core delivers chemical stability and a broad solvation range. Reports from established sources, including the Journal of Physical Chemistry B, routinely confirm these values across commercial samples.
Technical Specifications & Labeling
As supplied, 1-ethyl-3-methylimidazolium diethylphosphate typically comes with a purity exceeding 98%. The labeling includes its systematic name and the CAS number 648864-83-7. Labels clearly display water content, which affects its use for moisture-sensitive reactions—a crucial consideration, since ionic liquids can absorb trace humidity. Producers list the molecular weight (228.23 g/mol) and sometimes include conductivity and viscosity references for control labs. I have seen technical data sheets that highlight typical heavy metal content below 2 ppm, and reference storage suggestions: tight sealing, low humidity and ambient temperature.
Preparation Method
The main commercial process begins by reacting 1-ethyl-3-methylimidazolium bromide with sodium diethylphosphate in deionized water. The metathesis reaction produces sodium bromide, which then precipitates out as a white solid and allows for easy filtration. Filtration leaves a layer rich in [EMIM][DEP], which undergoes thorough washing before drying under vacuum. Research papers detail alternative synthetic avenues that avoid halides or use direct neutralization between imidazolium hydroxides and phosphoric acid esters, but the main route stays consistent because of its reliability and economic scale. Practice has shown that maintaining a controlled water content in the final product makes the difference in performance, especially when used for catalytic or electrochemical studies.
Chemical Reactions & Modifications
Chemists have tested [EMIM][DEP] in many reaction types thanks to its non-volatile solvent nature and ability to stabilize charged intermediates. It often supports transition metal-catalyzed transformations, where ligands and substrates remain soluble throughout temperature ramps. Researchers also documented the efficient use of [EMIM][DEP] in cellulose processing, dissolving natural fibers and enabling modifications without harsh acids. It does not oxidize or degrade when exposed to air, nor does it rapidly hydrolyze or produce dangerous by-products in typical lab conditions. Further modifications can tune its ionic strength or reactivity, but the backbone remains resilient to acid, base or moderate oxidation.
Synonyms & Product Names
Publications and catalogs list 1-ethyl-3-methylimidazolium diethylphosphate under several names: [EMIM][DEP], 1-ethyl-3-methylimidazolium diethyl phosphate, and its brand-labeled shorthand, EMIM DEP. Chemical Abstracts records hold 648864-83-7 as the most common CAS reference. Some suppliers use minor abbreviations, such as EMIM-diethylphosphate, but the essential information—identifying the unique imidazolium and phosphate combination—remains consistent. Researchers usually refer to it as “[EMIM][DEP]” in journals, reflecting both its positive ion and phosphate anion components.
Safety & Operational Standards
Safety data for [EMIM][DEP] comes from decades of lab work and a growing base of toxicological studies. Spill protocols shown in technical bulletins direct users to wear gloves and eye protection, since the liquid can cause mild skin or eye irritation after prolonged contact. Inhalation risk stays very low because of the absence of significant vapor at room temperature. Disposal protocols match those for many specialty chemicals: containment, labeling and transfer to chemical waste disposal streams, preventing direct discharge into soil or waterways. Most producers issue handling guides showing that [EMIM][DEP] remains stable when kept away from strong oxidizers or acids. The risk of fires, explosions or acute toxicity remains much lower than similar organic solvents, according to public records.
Application Area
Industry and academia both lean on [EMIM][DEP] for its capacity to dissolve polysaccharides, such as cellulose, which opens possibilities in textiles and biodegradable plastic manufacturing. Electrochemists value it for enabling interesting behaviors in batteries and capacitors, since it conducts ions well without evaporating and supports a stable voltage window. Catalysis literature shows [EMIM][DEP] holds a place as a medium for cross-coupling and transfer hydrogenation, making it a staple in synthetic organic chemistry. Environmental chemists look at its promise as a “green” alternative to volatile pollutants. Beyond the bench, [EMIM][DEP] makes inroads in pharmaceutical manufacturing and specialty materials, where manufacturers desire solvents that neither corrode nor contaminate reactors, and maintain product purity through multiple steps.
Research & Development
Researchers continue to expand the palette of reactions made easier by [EMIM][DEP], focusing on applications that benefit from its thermal stability and low volatility. Labs around the world work to optimize the processing of bio-based materials—such as delignification of biomass—opening routes to sustainable fuels and chemicals. At chemistry conferences, groups present findings on how tailoring the imidazolium or phosphate component changes performance for target applications, like increasing efficiency in cellulose breakdown or boosting selectivity in synthetic pathways. Electrocatalysis experiments show promise for energy storage devices and sensors, with tweaks in the cation/anion pairs fine-tuned for each purpose. Every year, more papers come out showing that the field hasn’t exhausted the options that ionic liquids offer.
Toxicity Research
Toxicity data for [EMIM][DEP] grows steadily as more groups publish both acute and chronic exposure studies. Early animal studies report low acute toxicity, far below that seen in conventional organic solvents, with LD50 values often above 2g/kg for oral exposure in rodents. Eye and skin irritation can occur, but so far, chronic health effects appear rare under current industrial and academic use patterns. Environmental researchers test the impact of accidental release in water, seeing moderate toxicity to aquatic organisms but less persistence compared to aromatic hydrocarbons. Risk assessment models recommend minimizing unnecessary exposure and ensuring wastewater is neutralized rather than released untreated. Safety protocols learned from earlier solvents mean that most facilities already have containment and cleanup in place for spills or accidental contact.
Future Prospects
As regulations on emissions tighten, the search for safer and more effective chemical technologies will push [EMIM][DEP] into new areas. The world wants less volatile pollution in the workplace, and the rise of electric vehicles and renewable energy amplifies demand for advanced materials and specialty electrolytes. Growing public attention on sustainable processing lines up with the strengths of this kind of ionic liquid, pushing further expansion in bioprocessing and recovery of rare earth materials. New R&D projects focus on reducing energy input during separation and recovery, making the most out of [EMIM][DEP] by combining it with new catalysts and solid supports. Continued work by life cycle analysts, alongside publication of more detailed safety data, will build confidence for wider industrial adoption, from food packaging to pharmaceuticals and energy storage.
An Ionic Liquid for Changing Industries
People who work with chemicals often run into 1-Ethyl-3-Methylimidazolium Diethylphosphate, usually known as EMIM DEP. This mouthful of a name belongs to a class called ionic liquids—salts that stay liquid at room temperature. Labs and factories prize this liquid for a good reason: it doesn’t evaporate or catch fire like most solvents do.
Powerhouse in Green Chemistry
EMIM DEP’s stability changes how people think about chemistry’s “green” shift. Flammable solvents can ruin the air and risk workplace safety. EMIM DEP doesn’t share those traits. It dissolves cellulose, which helps turn tough plant fibers into biofuels, biodegradable films, and even recycled paper. The old process used hazardous chemicals and left toxic leftovers. With EMIM DEP, the risk shrinks and the process uses less energy. Having worked with stubborn plant materials before, I know what a relief it is to have a solvent that won’t turn the lab into a hazardous zone.
Cleaner Energy and Batteries
Batteries shape energy for everything from cars to phones. The stuff inside—a mix of liquids and solids—needs to carry ions quickly and safely. EMIM DEP steps in as a safer electrolyte for lithium-ion and other batteries. It won’t blow up under heat or short-circuit the way standard liquid electrolytes have during nasty incidents in the news. Between safer labs and stable energy storage, there’s a direct link to homes and streets. Less risk in the factory means lower cost insurance and fewer safety recalls. That might not make headlines, but anyone who has paid for a battery recall knows why a stable solvent matters.
Smart Extraction and Industrial Uses
Pharmaceutical labs, metal processors, and recyclers want new ways to get valuable materials without using mountains of toxic waste. EMIM DEP pulls rare metals from junk electronics by separating what the industry wants from what it doesn’t need. Extracting cobalt, gold, or rare earth elements gets easier and safer. I’ve seen stacks of worn-out gadgets piling up in offices, and friends in recycling plants say every step toward less waste and fewer harsh chemicals helps keep jobs healthier.
What Makes It Safer — and Where Caution Matters
EMIM DEP doesn’t build up pressure or fumes, so storage and shipment stay less risky. Its chemical makeup resists sudden changes in temperature and pressure, lowering the odds of fires. Fewer emergencies—not just for lab techs, but for communities near factories—grow out of every step away from flammable solvents. Still, handling any chemical needs real care. Gloves, goggles, and good ventilation keep a useful material from becoming a health issue.
Where Change Starts Today
Regulations around clean manufacturing are tightening. EMIM DEP offers a shot at meeting those rules, but costs still run high and many smaller shops can’t afford such specialty solvents right away. Expanding production and recycling methods could cut prices over time. If more manufacturers push for greener options, and governments support research, EMIM DEP and liquids like it could turn complex chemical processes into cleaner, everyday routines. For now, those who handle lignocellulose, batteries, and rare materials see real value in this ionic liquid—and bring industry a step closer to safer, sustainable standards.
The Role and Make-Up of a Modern Ionic Liquid
1-Ethyl-3-Methylimidazolium Diethylphosphate—a bit of a mouthful—is a classic example of an ionic liquid. At room temperature, it stays liquid, which sets it apart from regular table salt or many other salts you find in the lab. I first crossed paths with this compound during a university project focused on alternative solvents. Back then, the buzz was all about these "green solvents" and their use in cleaner chemistry.
If you take a close look at the molecular structure, you’ll find a positively charged imidazolium ring paired with a negative diethylphosphate ion. This pairing helps keep the compound in a liquid state without the need for water. Such an arrangement gives it some key traits: low volatility, relatively high thermal stability, and the ability to dissolve a wide range of organic and inorganic materials. There was always something reassuring about handling a solvent that wouldn’t quickly disappear into the air or start a fire at the first sign of a flame.
Physical Features and Lab Life
Clear, colorless, and oily—those are the hallmarks of this liquid. It pours slowly, a reminder that its viscosity sits much higher than water. At typical room temperatures, it won’t freeze or burn up, since it can handle both higher and lower temperatures compared to many organic solvents. Measured densities often sit just over 1.1 grams per cubic centimeter—that’s a little heavier than water—so you won’t see it float to the top in a mixture.
In my own hands-on work, I found 1-Ethyl-3-Methylimidazolium Diethylphosphate to be nonvolatile, with negligible odor. This property alone offers a safety upgrade over traditional solvent choices. Fewer fumes means a more pleasant lab environment, and far less risk of inhalation hazards. You won’t need to dodge headaches from evaporating chemicals with this compound.
Chemical Personality and Practical Benefits
The imidazolium component acts as a stable base, while the diethylphosphate brings chemical versatility. This pairing does not let the liquid break apart easily under normal conditions. The molecule won’t get oxidized or reduced unless it meets pretty extreme environments. For chemists, that means it won’t react with air, light, or water in unpredictable ways. Stability translates to reliability.
I learned firsthand how useful this solvent becomes for dissolving things water can’t handle—the perfect fit for cellulose processing or catalysis. That’s not just hype; studies published in journals such as “Green Chemistry” and “Journal of Molecular Liquids” confirm that it works in biomass conversion, pharmaceuticals, and electrochemistry, without creating a pile of toxic byproducts. Ionic liquids like this one also show promise for use in batteries and other energy storage devices. Their thermal stability and broad solubility range offer real solutions for challenges in renewable energy tech.
The Real-World Challenge
Despite plenty of benefits, every choice comes with trade-offs. The high viscosity sometimes slows down reactions, so agitation and heat may be required for practical use. Another concern is cost—these specialized solvents add up quickly, especially for projects with tight budgets. Waste management and environmental impact must be handled responsibly, even if these compounds avoid the hazards of older solvents.
Green chemistry is still catching up in terms of figuring out recovery and reuse for ionic liquids. The key will be scalable, affordable recycling steps and ongoing testing to ensure no unforeseen toxicity issues show up later. The best solution hinges on collaboration between chemists, engineers, and policymakers, who all bring experience from their corners of industry and research.
Chemicals in Daily Life: What We’re Dealing With
Getting familiar with the technical names used in labs can seem daunting, but plain talk has its place. 1-Ethyl-3-methylimidazolium diethylphosphate belongs to a category we call ionic liquids. These chemicals turn up in everything from recycling processes to green chemistry experiments, offering a way to dissolve materials without the stink or volatility of old-school industrial solvents. But every promise carries a flip side, and understanding the hazards lets us walk forward with eyes open.
What Toxicity Really Means Here
Ionic liquids have gathered a reputation for “greener” chemistry thanks to low vapor pressure. This means less evaporation and fewer flammable fumes wafting around. Still, each chemical handles differently in the body and the environment. Looking at scientific databases and toxicity reports, 1-ethyl-3-methylimidazolium diethylphosphate shows low acute toxicity for humans. Short-term contact with the skin or eyes often causes irritation, but nothing worse if the exposure stays brief and limited. Swallowing or inhaling vapor might make someone nauseous or dizzy, the way other mild irritants might.
Brushing up on material safety data sheets gives a practical sense of risk. The chemical doesn’t act like strong industrial acids or bases—no instant burns or dangerous fumes in the air. Still, the worries do not just stop there. Some researchers have flagged that ionic liquids like this one can harm aquatic life. Even in small doses, fish and water bugs start to struggle. That sets off warning bells in my experience: even if workers in a factory wear gloves and face shields, run-off and spills can travel downstream. If not handled properly, these liquids add to a bigger problem in our rivers and lakes.
Why Care About Low-Level Risks?
Take it from anyone who’s cleaned up an office chemical spill—the dose still makes the poison. Even something called “low hazard” can stack up in bodies or environments over time. Some ionic liquids have shown persistence, lingering in soil and sediment. Research into these specific formulations remains ongoing, and the evidence isn’t all in yet. While nobody’s ringing alarm bells about mass poisonings, careful risk assessment is not the enemy of progress. We don’t want to swap one kind of pollution for another and call that innovation.
How Do We Work Smarter?
Labs that use 1-ethyl-3-methylimidazolium diethylphosphate need active ventilation, not just to cut down on any stray fumes, but to make people feel secure at work. Gloves and face protection should come standard when pouring or mixing. It never hurts to run through spill drills, since tiny accidents can escalate if someone panics or hesitates. Waste disposal matters even more. Dumping leftover solutions down the drain causes real harm to ecosystems. Secure waste storage and professional chemical disposal protect water sources and wildlife we seldom see from office windows.
Staying Ahead: Research and Responsibility
Scientists keep experimenting with “greener” chemicals for industry. Newer studies will clarify exactly how stubborn these liquids might be once they slip outside controlled lab settings. Until then, respect for hazard warnings and environmental care should drive daily choices. Choosing any chemical, even one with low risks on paper, deserves active attention—because accidents go easier on prepared minds and careful hands.
Why Respect in the Lab Matters
Chemicals have a way of teaching respect. Spend enough years around bottles with names like 1-Ethyl-3-Methylimidazolium Diethylphosphate and you’ll understand the importance of good habits. It’s easy to think, “This one looks safe enough”, especially for chemicals that don’t have strong smells or lurid warning labels, but that attitude leads to accidents or ruined samples. A small spill or careless exposure brings long-term consequences. In my early days, leaving a lab bench sticky and unclean forced us to toss pricey glassware and set back our project a week. Small details count.
Know What You're Handling
1-Ethyl-3-Methylimidazolium Diethylphosphate comes from the family of ionic liquids. Researchers like it for dissolving cellulose, extracting metals, or running green chemistry reactions. Even though this compound seems tame compared to something like hydrofluoric acid, it brings its own hazards—chemical burns, skin irritation, or dangerous mixtures if handled carelessly. The European Chemicals Agency gives it hazard statements, and its safety data sheet demands attention.
No Substitutes for Airtight Storage
Moisture messes with ionic liquids and changes their properties. I always put them in well-sealed glass bottles with PTFE-lined caps. Stash the bottle inside a desiccator or a dry cabinet, out of sunlight and away from heat, since UV and warmth often degrade sensitive chemicals. Don’t trust low-quality plastic, since chemicals like these can eat through weaker containers or leach contaminants. Tape a fresh label with the full name, date received, and the name of the tech who prepped it; mistakes happen when mystery bottles fill up a shelf.
Personal Protection Isn’t Optional
No one plans to get splashed, but it happens. Gloves—preferably nitrile—keep this compound off your skin. Safety goggles stop accidental splatters from burning eyes. I wear a cotton lab coat, which shields arms and chest. Most toxicologists warn against working with ionic liquids outside a fume hood, since tiny vapors or drops add up. I’ve seen co-workers forget their gloves and wind up with burns or rashes during cleanup. Caution becomes habit with practice.
Clean as You Go and Separate Solutions
Wipe down benches and wash equipment right after finishing your work. Even small drips become hazardous if the same compound reacts with acids, oxidizers, or gets on your skin weeks later. Store waste liquids in dedicated, labeled containers so there’s no guessing game during disposal. Never pour leftover solutions down the drain; call hazardous waste services or follow lab regulations designed for ionic liquids.
Talk and Train Constantly
Handing off a lab to new team members means walking them through real examples of spills or good storage practice. I keep a fresh copy of the SDS close by and quiz new staff regularly. Regulations change, so labs must review guidelines every year. It pays to build a culture where everyone speaks up about safety before mishandling leads to emergencies.
Building Habits, Not Taking Chances
Safe handling and careful storage of 1-Ethyl-3-Methylimidazolium Diethylphosphate isn’t about fear, but about respecting chemistry’s risks and rewards. Organized storage, protective equipment, and quick cleanup make the difference between smooth experiments and avoidable accidents. Years working in labs convinced me that attention to these details saves both time and skin.
Getting Your Hands on 1-Ethyl-3-Methylimidazolium Diethylphosphate
Tracking down high-purity chemicals doesn’t always feel straightforward, especially with specialty compounds like 1-Ethyl-3-Methylimidazolium Diethylphosphate. Most researchers and lab technicians I know head straight for the usual distributors—Sigma-Aldrich, TCI, Alfa Aesar, and Merck. These companies have global reach, meaning you’re likely to find what you need whether you’re in the US, Europe, or Asia. If you’re working with a university or a corporate lab, your procurement team probably turns to these catalogs first. Online chemical marketplaces like eMolecules and ChemSpider also connect buyers with suppliers and let you compare purity levels and prices.
Direct contact with the supplier always helps, especially when you need bulk quantities or custom specs. I’ve seen procurement officers call or email to negotiate lead times and check certifications. For those in regions without easy access to big names, local distributors handle import and paperwork, though prices jump with extra handling. Individual researchers without institutional backing run into more hoops—restricted shipping, requests for lab credentials, and extra scrutiny because of export regulations. Some suppliers refuse to ship to residential addresses or non-institutional buyers altogether.
Grades and Why They Matter
You’ll find 1-Ethyl-3-Methylimidazolium Diethylphosphate sold in several grades. Most suppliers offer at least a “research grade,” which covers the majority of academic experiments and basic industrial testing. If someone’s building sensitive devices or making pharmaceuticals, they look for “high-purity” or “analytical” grades, ensuring minimal interference from side products. Purity typically ranges somewhere from above 95% up to 99%, with certificates of analysis available if you ask. For a random synthesis project in our lab, 98% purity proved enough; no odd peaks popped up in NMR, and the yields stayed steady. If your protocol demands trace metal analysis or tight contamination control, contact the vendor. Some even share impurity profiles up front.
The food, biotech, and environmental sectors sometimes ask for “industrial” or “technical” grades, especially when purity isn’t the top priority. These grades trade off small impurities for better pricing and larger packaging sizes. Buyers often select grades based on compatibility with their own safety standards. From experience, I saw a waste treatment company spring for industrial grade to save costs, since their process handled small contaminants anyway. A pharmaceutical startup, on the other hand, insisted on analytical grade—they couldn’t risk bad batches or regulatory trouble.
Making Good Choices, Avoiding Pitfalls
Purchasing specialty chemicals never goes off gut feeling alone. Storage, stability, and labeling all impact the quality you receive. I always ask about recommended storage—you don’t want to discover months later that your bottle needs refrigeration or protection from light. Some dealers toss in a material safety data sheet, but it’s smart to double-check proper handling even if you’ve used similar ionic liquids before. I saw a colleague get burned—literally—by skipping gloves with what looked like a harmless salt. Safety protocols matter, especially for those working alone in smaller labs or startups.
Another issue I’ve seen is import restrictions. Even if a website lists a product in stock, customs can block chemicals due to regulations set by your country. It pays to talk with your supplier and shipping agent about forms, taxes, and delivery timelines. I’ve watched as shipments sat stuck in customs for weeks over missing paperwork. Planning helps avoid these headaches, especially with less common compounds like 1-Ethyl-3-Methylimidazolium Diethylphosphate.
Building Trust in the Supply Chain
Not every vendor treats quality and transparency the same way. Choose suppliers with clear documentation, batch consistency, and responsive customer support. Check for customer reviews, ask colleagues for recommendations, or look for third-party certifications. Some companies post recent batch analyses on their websites, making it easier to judge before you buy. In my experience, working with a reliable supplier reduces hassle and keeps projects moving.