1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate: A Deep Dive
Historical Development
Researchers first paid attention to 1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate because the world wanted greener solvents and innovative materials for energy. The imidazolium-based ionic liquids appeared in the late twentieth century, springing from discoveries in organometallic chemistry and electrochemistry. Before this, chemists leaned heavily on volatile organic solvents for nearly every synthesis, but environmental and safety concerns kept growing. Several teams in Europe and Asia steered ionic liquids like this one from the back of the shelf into the main laboratory. Their low vapor pressure and unique ion structure invited researchers to think differently about making pharmaceuticals, processing cellulose, and creating cleaner industrial cycles. This wasn’t just about a trend; it was a change in the basic chemistry toolkit.
Product Overview
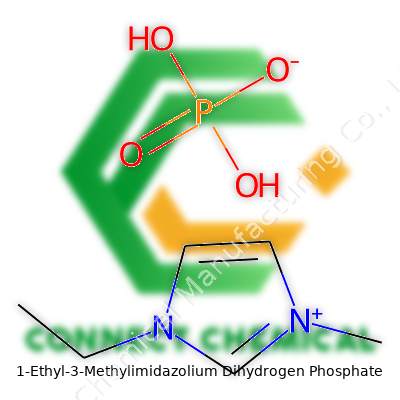
Across the last two decades, 1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate, often called [EMIM][H2PO4], showed up on material data sheets for use as a solvent, a catalyst, and an electrolyte additive. Industry and academia both look at the compound as a workhorse for tasks that used to grab at toxic or flammable chemicals. The molecular structure brings together a positively charged imidazolium ring with a dihydrogen phosphate anion, striking a balance between stability and reactivity. It usually presents as a clear to pale yellow liquid at room temperature, one of those compounds you could run a finger across in the fume hood without feeling the sting you'd get from older industrial solvents.
Physical & Chemical Properties
EMIM dihydrogen phosphate weighs in at a molecular mass close to 222 g/mol. This liquid rarely carries an odor, doesn’t evaporate into the room air, and feels slightly viscous to the touch. It dissolves well in water and polar organic solvents, filling a niche where traditional ionic liquids can't handle high moisture because of hydrolysis concerns. Its density sits above water, typically around 1.2 g/cm3. The melting point drifts below standard room temperatures, explaining why it flows easily in lab glassware. Heat it up, and it stands firm up to about 200°C before decomposition. Not so many chemicals cover both thermal stability and solubility in water, offering a surprisingly gentle chemical profile compared to the harshest acids and bases.
Technical Specifications & Labeling
The chemical industry sells EMIM dihydrogen phosphate at purities between 95% and 99%, packing it in amber glass bottles and heavy-duty fluoropolymer containers to block any reactive leaks. Labels show the full IUPAC name, batch number, and manufacture date, plus all pertinent safety phrases. Its CAS number—616817-66-0—makes cross-referencing easy in digital inventories. The product safety data sheet highlights potential hazards, environmental handling, and recommended first aid. Most suppliers impose rigorous purity analysis by NMR, IR, and ion chromatography, nailing specs for research and high-performance industry processes.
Preparation Method
Chemists make EMIM dihydrogen phosphate by first crafting the EMIM cation. This step starts with methylimidazole and ethyl bromide, a classic quaternization step that forms EMIM bromide under mild heating. Then, through metathesis, the bromide ion swaps places with dihydrogen phosphate. By adding phosphoric acid and neutralizing in a salted environment, the dihydrogen phosphate pairs tightly with the imidazolium cation, driving out impurities with washes and vacuum drying. Tweaks to the process minimize halide residues and ensure no leftover acids lurk in the product—important for downstream reactions or sensitive equipment. Companies producing larger batches use controlled temperature and pH equipment, automatic mixers, and sealed reactors to keep consistency up and contamination down.
Chemical Reactions & Modifications
One key feature is its resilience in acid-base conditions. As a solvent, EMIM dihydrogen phosphate supports various organic couplings, esterifications, and oxidations without itself breaking down. The phosphate group can moderate reactions—sometimes acting as a mild acid or a weak base. In certain setups, researchers swap the phosphate anion for other functional groups, producing ionic liquids tailored for extraction, catalysis, or even gas capture. This ability to change anions while stabilizing the imidazolium cation puts EMIM derivatives in the running for custom-designed materials. In electrolytic cells, the phosphate can participate in proton conductance, making the compound valuable for fuel cell research and batteries.
Synonyms & Product Names
The literature refers to this compound as EMIM DHP, EMIM(H2PO4), or simply the longer name without abbreviations. Suppliers list slight variations, usually shorthanding the cation and anion for cataloging speed. Academic papers sometimes use “1-ethyl-3-methylimidazolium phosphate” to save space, but precision matters for reproducibility. No matter how it’s labeled, the imidazolium core and the phosphate counterion set this chemical apart from the wider crowd of ionic liquids.
Safety & Operational Standards
The low volatility of EMIM dihydrogen phosphate means much fewer headaches about inhalation compared to traditional organics. Still, direct skin or eye contact produces irritation, so gloves and goggles aren’t optional. Laboratory safety data call for splash protection, ventilation, and good housekeeping to protect staff and students. Ingestion, though rare, brings risk because ionic liquids remain biologically active; standard protocols call for rinsing and medical attention if exposure occurs. Waste disposal routes it through chemical waste streams, usually handled by neutralization and incineration, avoiding dilution down the drain. Documentation stays current, integrating new environmental and toxicology findings as research turns up more information.
Application Area
Industry leans on EMIM dihydrogen phosphate for dissolving cellulose, spinning new fibers, and processing plant biomass. The textile field experiments with the compound for more sustainable fiber creation, leaving behind harsh chemicals from the old school. Pharmaceuticals investigate its role as a reaction medium for challenging syntheses or selective extractions. Energy labs use it to make safer electrolytes for batteries or as a proton carrier for fuel cells, where old-school acids don’t cut it. It’s also drawing attention as a catalyst and stabilizer in green chemical syntheses—a move that fits ongoing efforts to clean up downstream manufacturing and reduce environmental footprints.
Research & Development
Experts track EMIM dihydrogen phosphate in hundreds of new articles, with big journals publishing studies on its effects in electrochemical cells, extraction techniques, and catalysis. R&D teams follow its capacity to process renewable feedstocks, substitute for volatile organic solvents, and improve component longevity in energy storage systems. The adaptability of the imidazolium structure keeps labs returning to this family, trying out tweaks for stronger performance or better selectivity. Efforts to recycle and recover ionic liquids turn up in the literature—no one wants to swap an old pollution problem for a new one.
Toxicity Research
Research into the toxicity of imidazolium-based ionic liquids reveals a mixed story. While EMIM dihydrogen phosphate ranks safer than many classic organic solvents, it does show moderate toxicity to aquatic organisms in concentrated form. Studies in cell cultures and animal models keep an eye on possible enzyme or organ effects over long exposure times. Regulations push manufacturers to limit environmental release and follow strict workplace concentration limits. As usage grows, researchers keep testing for long-term, low-level impacts, filling in knowledge gaps to keep both workers and water sources safe.
Future Prospects
EMIM dihydrogen phosphate stands on the edge of even broader adoption across industries bent on greener solutions. The chemical’s flexibility opens new routes for biomass conversion, energy storage, and high-selectivity catalysis. Investments into closed-loop manufacturing, where solvents recycle rather than disappear, position ionic liquids favorably for next-generation material production. As new processes and safety standards develop, expect to see EMIM dihydrogen phosphate play a bigger role in the march toward sustainable chemistry. Both regulatory bodies and marketplace competition will demand transparency and continued toxicity evaluation for any expansion, but momentum keeps growing for ionic liquids that blend performance with responsibility.
A Closer Look at the Chemical
1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate—unwieldy to say, yes, but it’s a standout among ionic liquids. In laboratories and factories where chemical engineers and scientists look for cleaner, greener ways to do tough jobs, this material keeps popping up. I’ve seen it spark interest not just on paper, but in settings where folks hunt for alternatives to fossil-fuel-based chemicals and less-healthy solvents.
Powering Greener Chemical Processes
Traditional solvents like toluene or acetone release fumes and carry health risks. The shift toward safer, more sustainable options lands ionic liquids in a sweet spot. 1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate acts as a solvent in reactions that demand stability and safety. It doesn’t vaporize easily, cutting down risks tied to inhalation or explosion. That’s a relief both for the people mixing chemicals and for anyone nearby.
In one industrial setting, I saw chemists use this compound to break down cellulose from plant matter. Old methods left behind toxic byproducts, but this ionic liquid handled the job at a lower temperature and left little waste. This helps companies chasing biofuels or bio-based plastics make products that don’t cost the environment so much.
Battery and Energy Storage
Energy storage gets more attention every year. Lithium-ion batteries, the same kind in your phone or electric car, rely on electrolytes that can get hot or catch fire. 1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate steps in as a safer, non-volatile electrolyte. Its thermal stability shines in heat-intensive situations, so batteries run longer, with much less risk of something catastrophic. This chemical helps edge us closer to better, safer batteries for solar power, grid storage, and electric vehicles.
Catalysts and the Push for Cleaner Polymer Production
Plastic production faces fresh demands for less waste and fewer harsh chemicals. Some polymerization reactions need catalysts that work in rough conditions, and this is where this ionic liquid shows its value. By offering a medium that dissolves a variety of reactants, it acts as a reliable partner in polymer synthesis. Plants experimenting with eco-friendly plastics mix this liquid into their process to keep output high without choking on regulations or waste disposal headaches.
Advanced Applications: Biomass Conversion
I once watched a research team choose this compound for its ability to process tough plant fibers. Breaking lignocellulose into sugars often means harsh acids and high temperatures. This ionic liquid goes easier on equipment and workers. The clear result—cheaper, cleaner access to sugars needed for green fuels or specialty biochemicals. This single change can knock down the economic barriers holding back next-generation fuels.
Navigating the Hurdles
Cost and reuse stand out as the big hurdles. Ionic liquids have never come cheap, at least not in bulk. Recovery and recycling methods continue to improve, but industry still asks for better. What keeps momentum is the push from clients asking, “How can we go greener?” and the demand for products not shackled to old polluting models. Companies teaming up with researchers make progress, but the price, purity, and availability all shape which projects move fastest.
Paving the Way Forward
This material doesn’t fix everything overnight. It does what good technology should—it opens new options and chips away at limits. 1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate brings a safer, smarter toolset to chemistry. I’ve watched it turn skepticism into enthusiasm once the safety reports and emissions figures hit the floor. Real change depends on finding the right balance of performance, cost, and safety, which is exactly where this ionic liquid keeps earning a second look from professionals who need real, working solutions.
Understanding the Chemical in Everyday Contexts
1-Ethyl-3-methylimidazolium dihydrogen phosphate turns up in more research labs these days. It falls into the category of ionic liquids, which means it flows like oil rather than acting like a typical salt. People use it in green chemistry projects, batteries, and advanced materials. With new chemicals like this, clear guidance matters—especially for safe handling and storage.
Getting Hands-On: What’s Smart in the Lab
Most folks working around this chemical wear gloves and safety glasses, and that basic precaution goes a long way. The reports say this compound can act as an irritant—get a splash on your skin or eyes, you’ll know it. Some people dive into technical data sheets, and these tend to highlight similar advice: limit skin contact, avoid breathing dust or vapors, and keep food or drinks out of the lab. I learned quickly during my student years that trading comfort for proper barrier protection never turns out well. Once, I underestimated the residue from a similar ionic liquid, and even after a quick rinse, my skin was annoyed for a full day. Nothing dramatic, but a reminder not to get lazy.
Facts on Risks and How They Stack Up
Many ionic liquids get praised for low volatility and non-flammability. On the surface, this means fewer worries about inhaling dangerous fumes or sudden fires. Dig a little deeper, and you start noticing a concern: waste handling. If it spills, the absorbent pads and double-bag disposal routine come out. Most safety sheets push for well-ventilated spaces and the use of chemical fume hoods. The Environmental Protection Agency still classifies many ionic liquids as having unknown long-term effects, especially for aquatic environments if dumped down the drain. Hearing colleagues stress over proper waste containers is common for a reason—dumping it in the sink may feel easy, but that storm often comes back on the laboratory.
Storing for Success and Why It’s Worth the Trouble
Keeping 1-ethyl-3-methylimidazolium dihydrogen phosphate in sealed, chemically-resistant containers sits high on most lab protocols. Humidity often breaks down sensitive compounds. Cool, dry storage avoids unwanted reactions. Temperature swings stress chemicals in ways that can’t be reverse-engineered if something starts decomposing. Storing this compound away from acids or bases guards against surprise reactions. Having seen old bottles creep into strange colors, I know firsthand that “Do Not Store Near Reactive Chemicals” means what it says.
Paths Forward: Building Better Habits
Working with chemicals like this doesn’t call for panic—just a respect for what you’re handling. Real-time labeling, tidy storage, and using the right gear make a difference, even if it slows things down at the end of the day. For facilities with rotating staff or student researchers, holding regular walk-throughs keeps folks updated and cuts down on mistakes. As more labs try ionic liquids like this one, swapping stories and safety tips helps avoid repeating old missteps. Anyone using these compounds owes it to themselves and coworkers to keep safety habits sharp, even after the thrill of new research starts to fade.
Chemical Purity Sets the Standard
Not all chemicals come packaged with the same level of quality. Purity levels mean everything when it’s time to hit the lab or production line. Purity tells us how many impurities are hanging around – those trace elements, moisture, or other surprises that can throw off results or gum up a process. Over the years, I’ve seen how a difference between 98% and 99.9% can change the outcome. For example, lab work involving analytical analysis won’t tolerate anything but high-grade, research-level purity.
On the other hand, a plant working on large-scale production isn’t always chasing those last decimal points. Cost steps in, and sometimes the slightly lower grade still checks all the boxes. A pharmaceutical company never rolls the dice on purity because each microgram gets scrutinized. A manufacturer making paint or detergents might set their sights elsewhere, drawing the line at technical grade or industrial grade—good enough for the job, easy on the wallet.
Purity Grades in Practice
Let’s break it down. Chemicals usually come labeled with grades such as technical, laboratory, research, or ACS. ACS means American Chemical Society grade, which sets a high bar—typically around 99% or better for most compounds. Lab grade often runs just a notch lower, suitable for teaching labs and processes without critical thresholds. Technical grade, widely seen in industry, will usually have the least strict thresholds for contaminants and by-products.
Chemists, scientists, and industrial buyers need to check the certificate of analysis and SDS before ordering anything. Savvy buyers know that one company’s “high-purity” can include impurities another supplier would never allow. Ask for documentation. If that information is missing, that’s a red flag. I’ve always told my team: no paperwork, no purchase. Skipping this step has cost companies in lost batches, downtime, and regulatory headaches.
Packaging Sizes: Options Built for Real Workflows
Packaging options have grown as needs change. Small-scale labs might look for bottles as small as 100 grams or 500 milliliters. Those working with big processes count on tubs, drums, or even bulk tankers. There’s a practical side here—smaller packages control loss and cross-contamination, but large drums and super sacks drive down per-unit cost and simplify logistics.
Some suppliers stick to the basics: 500 g bottles, 5 kg tubs, and 25 kg bags. For solvents or high-use reagents, 200-liter drums or even 1,000-liter IBCs keep production humming. I once worked with a team buying sodium hydroxide by the truckload; the warehouse stocked 20-ton tankers rolling in every other week. Not everyone needs those volumes, but the key is asking suppliers for what really fits your workflow, not what’s listed on a spec sheet.
The Role of Documentation and Trust
Verified documentation builds trust and helps users feel confident that what’s inside the container matches the label. Regulatory audits, particularly in pharma and food, can pull documentation at any moment. Inconsistent packaging or vague purity claims invite trouble. For many shops, good supplier relationships mean quick access to certificates, test results, and reliable customer service when a question pops up. Keeping an open line to your distributor or manufacturer helps head off supply chain hiccups.
Finding Solutions That Work
Chemical users face serious headaches when purity and packaging don’t line up with their needs. Two real fixes: build supplier partnerships and stay current on documentation. Switch suppliers if traceability weakens. Push for regular updates on packaging innovations. Some companies now offer smart labeling or tamper-evident seals, giving another layer of confidence. Staying involved and asking questions keeps operations safe and compliant—and keeps budgets on track too.
Looking at the Promise and Reality of Ionic Liquids
Green chemistry never stops searching for answers to cleaner, safer chemical practices. Over the years, the rise of ionic liquids has sparked hope across the world of synthesis and separation science. They often promise low volatility, high thermal stability, and, sometimes, even a lower environmental footprint. Still, with every new candidate for these jobs, we have to check whether performance matches the promise—especially when the goal is to avoid replacing one problem with another.
1-Ethyl-3-Methylimidazolium Dihydrogen Phosphate: A Closer Look
If you land in a research lab focused on solvent alternatives, there’s a good chance someone has a vial of 1-ethyl-3-methylimidazolium dihydrogen phosphate on the bench. Its mouthful of a name gets slimmed down to [EMIM][H2PO4]. It comes handed down with a reputation for relative non-volatility, decent ionic conductivity, and an ability to dissolve a wide range of substances, including cellulose and metal ions. For green chemistry people—especially anyone who’s stirred up noxious fumes in a traditional organic synthesis—this sounds like a small miracle.
The experience of swapping out a volatile organic solvent like dichloromethane for an ionic liquid such as [EMIM][H2PO4] translates to fewer headaches (literally and figuratively). It doesn’t stink up the place, and you’re not left wondering whether you’re quietly leaking greenhouse gases into the air every time you open a bottle.
Moving Beyond Hype: Digging Into the Environmental Profile
Eco-friendliness is about more than just a substance not evaporating. Some early enthusiasm for ionic liquids faded once new research showed that not all of them live up to their green label. For example, toxicity and the fate of these compounds in water and soil show up as real concerns. In the case of [EMIM][H2PO4], studies suggest it’s less toxic to aquatic organisms than some other ionic liquids, though it’s not without risk. The imidazolium cation tends to be more biodegradable than many fluorinated alternatives, which lines up with efforts to cut down on persistent pollutants.
From my pragmatic view in the lab, waste handling ends up carrying just as much weight as initial solvent choice. Ionic liquids usually cost more, and that adds pressure to recover and recycle them. [EMIM][H2PO4] suits recycling pretty well under many reaction conditions—but there’s no guarantee, especially if the chemistry gets aggressive or lots of side-products creep in. Developers need robust methods for purification and reuse. Nothing ruins a green chemistry win like barrels of contaminated “liquid waste” you can’t deal with safely.
Charting a Smoother Path Forward
For [EMIM][H2PO4] to really excel, chemists should weigh the full process, not just swap out one solvent for another based on one attractive property. Emphasizing biodegradability in design, simplifying recovery, and ensuring end-of-life doesn’t create new disposal headaches should become routine. Transparent toxicity testing and honest assessments can protect against greenwashing. Industry and academia lose trust if slogans outpace safety data.
From my own work screening solvents, I’ve learned the best options often come from pairing cool new materials with old-fashioned stubbornness about testing everything—shortcuts only show up later as unnecessary costs or toxic surprises. [EMIM][H2PO4] carries promise. Like any “green” tool, it does its job in the context of a system, not just at a benchtop or in a conference presentation. Asking the tough questions up front saves everyone time—and spares plenty of awkward newsletter retractions down the road.
Facts: CAS Number and Molecular Formula
Every chemical on the planet tracks back to a unique ID―that’s the CAS number. For 1-Ethyl-3-methylimidazolium dihydrogen phosphate, this number is 270876-35-6. You spot this label in scientific literature, on chemical bottles, and across supply chains. It’s more than a code; it keeps everything consistent when folks in labs, factories, or universities compare notes or buy supplies. The molecular formula tells its own story: C6H13N2O4P. These letters and numbers spell out the building blocks of the compound and leave no ambiguity for researchers, regulators, or anyone working with the stuff.
Why This Matters Beyond the Lab
From time spent tutoring chemistry students and consulting with small manufacturing operations, it’s clear that access to the right chemical information can make or break a project. Companies depend on accurate IDs to follow safety regulations and prevent dangerous mix-ups. Science teachers and undergrads need to call up the right structural diagrams and hazard data. If someone grabs a compound with a similar-sounding name but a different CAS number, the results can swing from disappointing to downright risky.
A Tool for Innovation and Responsible Handling
This specific ionic liquid—1-Ethyl-3-methylimidazolium dihydrogen phosphate—shows up in a range of cutting-edge work. Researchers look at it for green chemistry, alternative solvents, and electrolyte development. It’s taking on roles in cellulose processing, catalysis, and even waste treatment. People drawn to this compound often have environmental and economic reasons behind their choice. Ionic liquids like this one often lower VOC emissions and open up routes to improved recyclability, since they won’t evaporate like traditional solvents. None of that progress happens safely without clear identifiers.
Transparency and the Global Chemistry Supply
A world built on chemistry can only hold together with trustworthy data. Too many times, small labs and startups run into delays because a supplier posts the wrong formula, omits the CAS number, or otherwise muddles the paperwork. Those mistakes put research on hold, burn budgets, and undermine confidence in the global supply chain. Regulatory agencies like OSHA and REACH rely on standardized chemical information to set workplace exposure limits, maintain shipping safety, and enforce bans or restrictions where health risks show up.
Building Better Access and Safer Labs
People handling, labeling, or transporting hazardous materials benefit from education and cross-checking CAS numbers. Many universities and workshops now include rigorous chemical inventory management in their lab safety courses. Mobile apps and online platforms let researchers scan a barcode and immediately confirm CAS, formula, and safety guidelines. These data-driven tools can stop mistakes at the door and cut down on accidents or wasted resources.
Room for Improvement: Transparency and Open Data
One big step forward would see chemical suppliers, distributors, and educators putting more of these hard facts—CAS numbers, precise molecular formulas, SDS sheets—front and center in catalogs, research papers, and product listings. This shift stands to save scientists hours, prevent regulatory fines, and build real trust between buyers and sellers.
Every field depending on chemicals advances more smoothly with this kind of clarity. 1-Ethyl-3-methylimidazolium dihydrogen phosphate stands as a reminder that attention to detail delivers results, keeps people safe, and fuels responsible discovery.