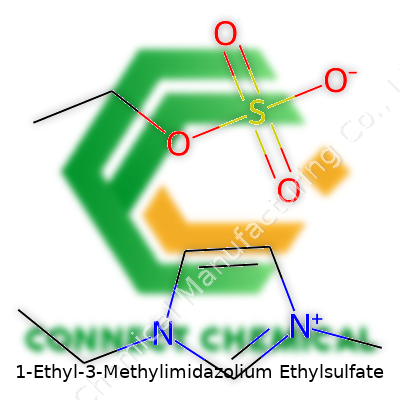
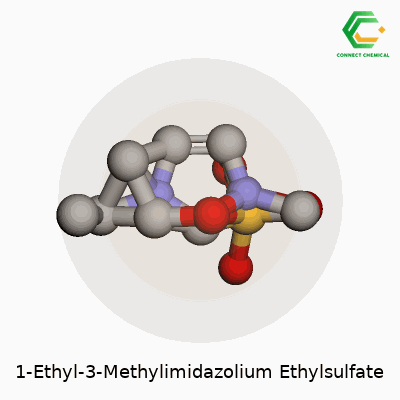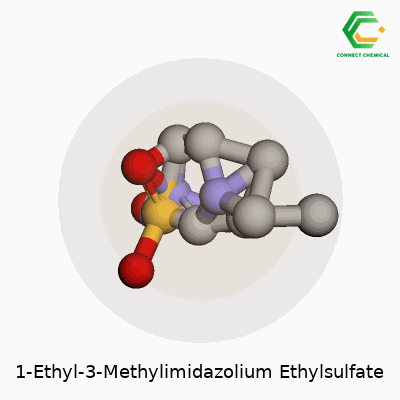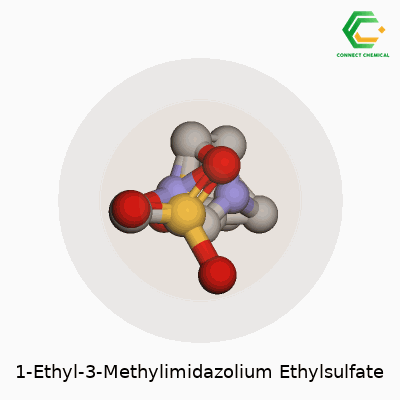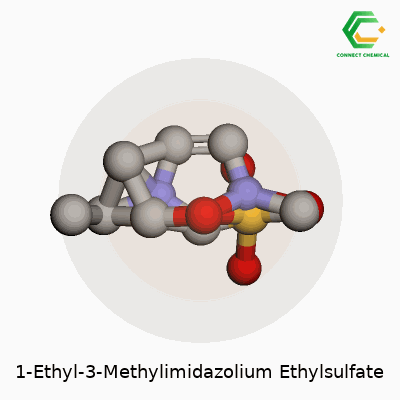
1-Ethyl-3-Methylimidazolium Ethylsulfate: A Keen Look at an Industrial Workhorse
Historical Development
Chemistry’s progress rarely follows a straight path, and the story of 1-Ethyl-3-Methylimidazolium Ethylsulfate—a substance known in labs for its handy initials, [EMIM][EtSO4]—illustrates this well. Born from the broader family of ionic liquids, it came out of late-20th-century projects looking for alternatives to volatile organic solvents. The imidazolium core didn’t get much attention in the early 1900s, but by the 1980s and ‘90s, researchers started mixing these cations with an impressive variety of anions. The resulting combinations shrank environmental impact and cut down on air pollution, opening doors for less hazardous chemical manufacturing. Backed by pioneers in green chemistry and aggressive pharmaceutical research, [EMIM][EtSO4] carved out a place for itself thanks to favourable solubility and stability.
Product Overview
[EMIM][EtSO4] looks unremarkable at first glance: a clear to yellowish liquid that barely gives off an odour. Behind the boring appearance lies a powerful solvent ready for action in tough reaction conditions. Suppliers might weigh it, pack it into sturdy containers, and slap on a simple product code, but inside those bottles sits a key ingredient in chemical transformations ranging from cellulose processing to advanced catalysis. Modern labs appreciate its low volatility, which allows work in open-air setups without clouds of harmful fumes.
Physical & Chemical Properties
Pick up a material data sheet and [EMIM][EtSO4] practically shouts versatility. The melting point usually sits below room temperature, keeping it liquid in most settings. Its density often lands around 1.24 g/cm³ at 25°C, so it feels heavier than water but not overwhelmingly thick. Water solubility comes naturally, and that means it mixes well with both polar and non-polar compounds. Unlike traditional solvents, it resists evaporation—thanks to negligible vapour pressure—cutting down atmospheric release. Thermal stability walks hand in hand with electrochemical steadiness: it doesn’t catch fire easily and resists breaking down under typical lab heat or minor voltage swings. These features have saved engineers headaches during sensitive syntheses.
Technical Specifications & Labeling
Labels on [EMIM][EtSO4] matter for more than compliance: precise identification means safe handling and effective downstream work. High purity—often above 98%—lets users skip extra purification and head straight for their reaction flask. Labels usually mention the batch number, date of manufacture, water content (important for controlling reaction sensitivity), and the identity of the anion and cation. Volume or mass, hazard pictograms, and supplier contact details round out the information. Clear markings help folks avoid confusion and accidents when storing with other ionic liquids that might look similar at a glance.
Preparation Method
Production relies on relatively straightforward chemistry but needs careful execution. Start with 1-Methylimidazole and 1-Ethylsulfate in a reaction vessel, introduce a solvent such as acetonitrile or another compatible medium, and heat gently while stirring. After the reaction, cooling and systematic washing reduce byproducts and purify the solution. Evaporation or rotary distillation pulls out volatile traces, leaving behind the prized ionic liquid. Any water content remaining gets trimmed away by vacuum drying or treatment with drying agents. Industrial setups scale this process by linking reactors in sequence, but the logic on a larger scale follows the same rules as a benchtop.
Chemical Reactions & Modifications
[EMIM][EtSO4] demonstrates resilience to most non-oxidizing conditions, so it partners well with strong acids or bases in multi-step reactions. It supports catalytic cycles, especially in homogeneous transition metal catalysis, where the ionic nature aids solubilization and even sometimes enhances catalyst lifetime. Techniques like alkylation, transesterification, and nucleophilic substitution happen in its presence without aggressive solvent waste. Modified versions emerge by swapping out the ethyl group or the imidazolium ring, giving researchers hundreds of variants designed for tasks like CO2 capture, pharmaceutical synthesis, or polymer processing. This adaptability fuels ongoing research aimed at finding even cleaner solvents and task-specific ionic liquids.
Synonyms & Product Names
This compound responds to several aliases, each giving a hint about its family tree. Common names include 1-Ethyl-3-methylimidazolium ethyl sulfate, EMIM EtSO4, and [EMIM][EtSO4]. Large chemical suppliers sometimes use product codes or catalogue entries alongside these names, which aids cross-referencing in multinational projects. These synonyms span technical documents, import/export ledgers, and academic research, helping bridge the language between commercial, regulatory, and lab-based work.
Safety & Operational Standards
Workplace safety plans classify [EMIM][EtSO4] under substances requiring gloves, eye protection, and working fume extraction. Direct skin or eye contact causes irritation, and accidental inhalation or ingestion, although rare, invites health problems. Standard storage keeps containers tightly sealed and away from oxidizers or acids, since reactivity climbs quickly near these substances. Waste takes a trip to chemical collection points or incinerators rather than drains. That means any operation using this liquid builds protocols around chemical hygiene, limiting risks and keeping both people and the environment unharmed. In my own research, attention to these rules prevented avoidable spills and saved hours of emergency cleanup.
Application Area
Industrial chemistry trusts [EMIM][EtSO4] where tough solubilization or high reaction speed are valuable. Paper factories dissolve cellulose for easier shaping and engineering designers use it as a solvent or electrolyte in specialized batteries. Labs chasing new catalysts often look to this compound for consistent, repeatable data. It also joins enzyme chemistry, where it helps stabilize delicate proteins during experimental testing. In practical life, these contributions land in products like sustainable plastics, new forms of insulation, and even advanced pharmaceuticals. From bench-scale to pilot plants, having a solvent that refuses to evaporate too soon or interfere with reactions makes all the difference.
Research & Development
R&D for [EMIM][EtSO4] explodes with new directions every year. Scientists probe its ability to recycle catalyst mixtures, seeking savings and greener approaches. Teams in bioengineering use it as both a medium and modifier for breaking down plant matter, aiming to make biofuels cost-effective and carbon-friendly. In the energy sector, research tracks how [EMIM][EtSO4] manages charge-carrier movement in next-generation batteries and supercapacitors. Integrating this ionic liquid into automated chemical reactors speeds up repetitive experiments and produces cleaner, more reliable data. Every time a team tweaks its cation-anion pairings, they uncover fresh reactivity and better adaptation to unusual substrates.
Toxicity Research
The growing body of toxicity studies keeps industry and academia balanced between potential and responsibility. Recent assessments track metabolites and measure toxic effects in both aquatic and soil organisms. Researchers investigate bioaccumulation and chart possible impacts across ecosystems if a spill occurs. Comparing results to conventional solvents, [EMIM][EtSO4] often shows lower volatility-related dangers, but questions remain about longer-term persistence and breakdown products. Regulatory agencies monitor usage and call for continued transparency in disclosing both human and environmental testing. Cautious optimism drives innovation toward even safer ionic liquid alternatives, informed by high-quality, peer-reviewed studies and real-world monitoring.
Future Prospects
Expectations for [EMIM][EtSO4] stretch into advanced materials, smart energy systems, and high-throughput chemical manufacturing. The current pace of automation and green chemistry supports further expansion, as this solvent matches emerging standards for sustainability. Creative partnerships between research institutes, startups, and established manufacturers funnel new applications into energy storage, sensor technology, and recycling. Developing nations could benefit from processes that replace older, hazardous chemicals with cleaner ionic liquids. If the field continues to prioritize practical safety, environmental review, and scalable production, [EMIM][EtSO4] will keep playing a central role in chemical progress—constantly evolving to meet the world’s shifting needs.
What Makes This Ionic Liquid Special?
1-Ethyl-3-Methylimidazolium Ethylsulfate seems like a mouthful at first glance, but its uses keep popping up in more and more fields. This ionic liquid belongs to a group of chemicals that don’t evaporate like regular solvents. Over the last decade, research and industry started paying more attention to it because it solves a lot of problems that come with traditional chemicals.
Boosting Green Chemistry in Labs and Factories
Companies and universities always look for safer materials that limit toxic fumes. This ionic liquid shows its strengths in that department. Unlike many organic solvents used for extraction or catalysis, it doesn’t burn off or pollute the air. I’ve seen labs use it to streamline tricky extractions—especially things like separating bioactive compounds from plants or purifying pharmaceuticals. The benefit goes deeper than cleaner air. This material keeps working after repeated cycles, which means less waste leaving the building.
Playing a Role in Better Batteries
The race to build stronger, safer batteries depends on finding the right ingredients. 1-Ethyl-3-Methylimidazolium Ethylsulfate handles ions well and resists breaking down, so manufacturers use it as an electrolyte inside lithium batteries and supercapacitors. It doesn’t explode or leak like some older materials, so safer designs become possible. Quality control teams appreciate that kind of stability—it makes devices last longer and charge better. Reliable tech like this builds trust with consumers.
Cleaning Up Pollution and Industrial Mess
Tackling tough environmental problems often involves taking toxins out of water or soil. Some researchers tested this ionic liquid and got strong results pulling heavy metals or dyes out of wastewater. Plants and aquatic environments rely on clean water just like people do, so replacing aggressive solvents with something safer matters at scale. It’s no magic fix, but efforts like these lower the health risks that follow industrial spills or poor waste management.
Helping Build Tomorrow’s Biomaterials
Biomaterials like cellulose have potential in everything from packaging to clothing. Traditional dissolving steps can be rough—using harsh acids or caustic soda that corrode equipment and harm workers. Labs that swap in 1-Ethyl-3-Methylimidazolium Ethylsulfate see gentler, more selective processing. The result is purer fibers or films with less chemical residue, which suits companies aiming for biodegradable products. This is where I see real promise for reducing reliance on plastics.
Pushing Scientific Knowledge Forward
Academic scientists use this material to model “green” reaction systems and study complex chemical processes that struggle with old-school solvents. There’s real value in a tool that lets you look closely at enzyme behavior or optimize medicine production steps. As journals keep publishing about it, I notice that breakthroughs in chemical engineering often start with safer, more flexible tools like this.
Room for Smarter Adoption
The key now is to keep evaluating safety, disposal, and economic factors. Labs and companies shouldn’t jump on new trends blindly. It helps to work with environmental engineers and share data about long-term risks or recycling options. Supporting policies and sharing best practices build a strong foundation for more responsible and efficient industry. I see promise for tools like 1-Ethyl-3-Methylimidazolium Ethylsulfate—especially when teams focus on both performance and stewardship.
Pushing Past the Labels: What Purity and Concentration Really Mean
Anyone who spends a Saturday scrubbing an aquarium or cleaning battery terminals knows that not all bottles of chemicals are created equal. Sometimes you spot a label shouting out “99% purity,” while another quietly claims 95%. These numbers do more than just look good on lab coats—they tell a story about what gets left out and how strong a given solution will perform. In my own garage, the stuff that promises higher purity gets stashed out of kids’ reach, because it packs a heavier punch.
The Lay of the Land: Purity Level
When we talk about purity, we’re really asking one simple question: “How much of this drum, bottle, or bag is the stuff we actually want?” Something labeled as 99.9% pure is nearly all active ingredient, with barely any fillers or unknowns. Chemists, pharmacists, and even folks in the food industry lean hard on that number. For example, pharmaceutical grade magnesium chloride boasts a purity higher than 98%, sometimes over 99%, because contaminants could spell trouble for patient health. Industrial grades land lower; sometimes in the 90-97% range, because it goes into things where a bit of dirt won’t wreck the outcome.
Purity testing uses tried-and-true tools like titration, chromatography, or spectroscopy, and those results end up on a Certificate of Analysis (COA) tucked away in a company’s paperwork. Having handled COAs working in a biotech warehouse, I always scanned straight for that purity level—one decimal point can decide if material ends up in a lab or just gets used for melting ice off sidewalks.
Concentration: More Than Just a Number
Concentration tells us how strong a solution or mixture is. In most chemical bottles, you’ll see concentration measured as a percentage (w/w, w/v) or in grams per liter. It means, for every 100 units—say, milliliters or grams—you know how much punch the stuff delivers. In the medical world, hydrogen peroxide for home use typically runs at 3%, but hospital-grade can reach 30% and more. That difference can hurt, literally, so seeing a bottle labeled clearly is not just paperwork—it’s the difference between a mild antiseptic and a chemical burn.
For folks in the lab or out on the factory floor, having the right concentration cuts down on waste and accidents. My time as a research assistant made me respect the fussiness—mixing up cell media, I measured everything down to the milligram. A small slip could mean scrapping hours of work. In agriculture, concentrated pesticides or fertilizers arrive in strong forms, so farmers dilute exactly as guidelines demand. When concentration strays, crops and rivers lose out.
Tackling Misinformation and Safe Access
Labels can confuse or mislead, especially in online stores. Some resellers fudge purity or concentration claims, leading to disappointed buyers or even accidents. The U.S. Food and Drug Administration keeps a watchful eye on many consumer chemicals, but the global market has cracks. It helps to look for reliable COAs, ask direct questions of suppliers, and stick to well-known brands. In my own household, I check for batch numbers and avoid anonymous imports for anything that goes near food or skin.
Safe handling comes down to trust—knowing that what’s inside the bottle matches what’s promised. For the professional or hobbyist, better oversight and transparency would take a lot of guesswork out of buying and using chemicals. Industry can step up its education efforts, and customers can insist on answers. In my own experience, getting the right facts on purity and concentration pays off not only in better results, but in fewer nasty surprises.
Why Safety Matters with Ionic Liquids
Safe chemical storage often makes the difference between an ordinary workday and a dangerous one. 1-Ethyl-3-Methylimidazolium Ethylsulfate (EMIM ES), a common ionic liquid, has earned a spot in many labs for its role in advanced materials and green solvents. EMIM ES stays liquid at room temperature, which helps with handling, but it still asks for respect. Like all chemicals, proper storage and handling help keep accidents at bay.
Real-World Storage Practice
Storing EMIM ES takes more than just shelving a bottle. It can absorb water from the air, which can mess up experiments or industrial uses. I’ve seen what happens when humidity sneaks in: contaminated product, lost time, and plenty of frustration. Keeping the bottle tightly sealed helps, but that only works if you use caps designed for airtight storage. Silica gel packs, tossed in with supplies, cut down on moisture risk. This chemical doesn't like sunlight either. Direct exposure can break down chemical bonds, so opaque containers or storage in a dark cabinet work best.
Temperature brings another factor. While EMIM ES stays stable at room temperature, extreme heat changes things — and no one wants a surprise reaction. Putting it away from furnace rooms or sun-facing windows usually keeps it out of trouble. Think about chemical neighbors, too. Strong oxidizers or acids hanging out nearby could spell disaster if they mix. A separate storage shelf, labeled, avoids this headache altogether.
Personal Protective Equipment: No Shortcut Here
Whether I’m pouring EMIM ES for an experiment or cleaning up a spill, I suit up with gloves, goggles, and a lab coat. Skin contact can cause irritation and chemical burns. Even a single drop is enough to teach you that gloves are worth the investment. I stick to nitrile, which stands up well to ionic liquids. Chemical goggles beat safety glasses for splash protection, another lesson learned after a small spill almost went toward an eye rinse station. Since inhaling any fumes isn’t the best idea, working in a fume hood or a well-ventilated lab pays off.
Spill Response: Fast Action Counts
Spills happen, even to careful chemists. Watching a peer panic during a spill drove home the need for a clear plan. Paper towels and water make a mess here, so I reach for absorbent pads rated for organic liquids. After soaking up EMIM ES, I seal the waste in a chemical-safe bag, label it, and tell colleagues right away. Cross-contamination sticks around when folks aren’t kept in the loop. Never forget to check the lab’s Safety Data Sheet for the chemical — it holds tips for managing accidental exposure or fire response.
Solutions for Safer Workplaces
Regular training drills, clear labeling, and honest communication about chemical hazards keep labs safer. Supervisors who walk the storage area once a week spot broken seals and worn-out labels before they become big problems. I once saw a lab overhaul its storage after a near-miss, and the cleaner, more organized shelves made work smoother for everyone. Having proper spill kits and the right PPE close by proves its worth every single time. Responsible habits, reinforced over time, matter more than high-tech gadgets or fancy alarms.
Understanding the Risks
Many labs and industries have turned to ionic liquids like 1-Ethyl-3-Methylimidazolium Ethylsulfate because they don’t evaporate into the air like volatile solvents. At first glance, this seems like a win for safety. The trouble is, their health effects haven’t been studied as thoroughly as old school chemicals, so operators can’t just assume they’re risk-free. I remember being in a research lab where students would describe “green solvents” and new materials as automatically safer. The lesson came fast: a clean-looking bottle doesn’t always mean safe.
This ionic liquid, sometimes called EMIM EtSO4, might sound modern and science-y, but it comes with its own list of concerns. Several peer-reviewed studies, including those published in the Chemosphere and Green Chemistry, point to harmful effects on aquatic life if spills occur near water. Even for those staying indoors, extended skin contact or inhalation of vapors from heating could cause irritation or worse, especially with repeat exposure. The compound’s oily texture can fool users into being less cautious compared to more pungent or caustic chemicals.
Personal Safety Experience in the Lab
People in academic and industrial settings sometimes develop lax habits as they get used to handling substances that don’t have strong smells or obvious warning labels. Years ago, I watched a colleague get a hand rash from not wearing gloves while measuring EMIM EtSO4 over several days. He figured it couldn’t be that bad since nothing looked hazardous on the bottle’s brief label. Cortisone cream helped, but it was a hassle that could have been dodged with basic protection.
In dozens of group safety meetings, the message always held steady: if you don’t know whether a chemical is dangerous, treat it with maximum respect. For this ionic liquid, the scientific literature recommends limiting contact and avoiding open containers near heat. Some reports suggest possible toxicity to fish at low concentrations, so any disposal protocol must keep it out of drains or groundwater. Fume hoods do more than protect from strong odors; they block tiny droplets and mists that could build up over time.
Protective Measures That Work
Several concrete steps can sharply cut down risks. Gloves should come standard; not the thin, clear food service type, but proper nitrile gloves that seal tightly around the wrist. Eye protection is more than a checkbox for an inspection. A single splash could cause serious harm, and cleanup is never as easy as it sounds in classroom demonstrations.
Lab coats or chemical-resistant aprons form a simple but tough barrier to accidental spills. Closed-toed shoes keep feet safe from drops on the floor. Using a fume hood for any process that involves heating or mixing really makes a difference. For storage, a sturdy, closed container, labeled in plain language—not just a formula or abbreviation—means nobody mistakes it for a less harmful liquid.
Why Vigilance Matters
Recent research continues to fill gaps in toxicity and environmental effects. Professionals in the chemical industry and academia owe it to themselves and their teammates to review updates on these materials. Trustworthy data comes from peer-reviewed journals and occupational safety agencies rather than casual internet forums. The risks may sound obscure, but a single incident, even a minor rash or eye splash, can change the way people work with chemicals for good. Respect for the unknown safeguards not only individuals but the entire workplace.
Rethinking Solvents for a Cleaner Planet
Traditional solvents in chemical labs fill the air with strong odors and leave behind waste that takes a toll on both people and ecosystems. My feet have stuck to floors coated in spilled acetone more than I’d like to admit. It’s tough to avoid these chemicals entirely—a lot of them get the job done effectively. But with green chemistry, the goal is not just better performance; it’s to stop pollution from the start. That brings us to 1-ethyl-3-methylimidazolium ethylsulfate, an ionic liquid with lots of promise, but not without drawbacks.
What Makes This Ionic Liquid Stand Out
I've watched researchers shift away from traditional organics—hexane, chloroform, toluene—because the impacts of leaks or spills don’t die off overnight. The first thing about 1-ethyl-3-methylimidazolium ethylsulfate is pretty striking: it barely evaporates at room temperature, and spills don't immediately send fumes across the lab. That cuts down on accidents and keeps working spaces healthier for longer stretches. Plus, it’s not flammable. I don’t worry about small heater mishaps turning into panic situations with this stuff around.
In green chemistry, reusability also counts for a lot. I’ve seen this ionic liquid survive cycles of extraction and separation without breaking down right away. That means less waste, less buying new chemicals, and fewer barrels heading off to the incinerator. Costs and risks both take a hit for the better.
Do the Benefits Outweigh the Risks?
It isn’t a perfect solution. Studies show 1-ethyl-3-methylimidazolium ethylsulfate takes time to biodegrade and can’t be called harmless if dumped down a drain. High concentrations can stress water life, and we just don’t have decades of data showing what happens once it escapes into the wild. That uncertainty gives me pause.
Production matters as much as disposal. Making this ionic liquid means using strong acids and sensitive processes, and the carbon footprint sometimes isn’t as low as the green label suggests. In practice, big chemical producers haven’t landed on consistent ways to keep manufacturing clean, cheap, and low-energy all at once.
Where It Fits in a Real Lab
I’ve sat in on conversations where using this ionic liquid solved headaches in cellulose processing or improved extractions from plant sources. It handles water and organic molecules side by side—a tough trick for most other solvents. Some labs recover and reuse nearly all of it, shrinking overall waste. Yet, for those smaller or less resourced operations, safe recovery isn’t always possible, so some of the green advantage gets lost.
Better Choices, Step by Step
With the pressure on to reduce chemical hazards, 1-ethyl-3-methylimidazolium ethylsulfate works in a pinch, especially if teams put in the effort to recycle and not overuse. Full adoption needs careful labeling, clear guidelines for safe handling, and strong policies about collection at the end of its life. As someone who’s dealt with chemical disposal headaches, I can say strong stewardship really matters.
No solvent is perfect, but using ionic liquids like this one as stepping stones could push the whole industry away from old-school toxics. Smart practices, patient science, and honest data-sharing—these help ensure every new “green” tool doesn’t just shift harm somewhere new.