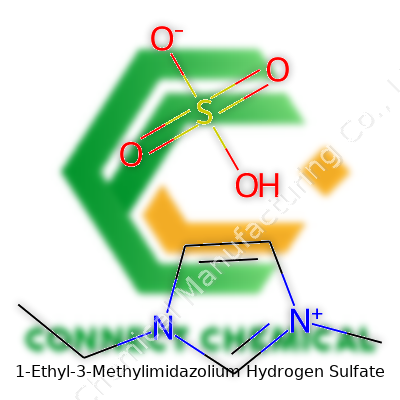
1-Ethyl-3-Methylimidazolium Hydrogen Sulfate: Navigating Chemistry’s Modern Frontier
Historical Development
Back in the 1990s, chemists started giving serious thought to alternatives to the old volatile organic solvents. Heavy regulations and environmental impact pushed research laboratories and industrial R&D teams to mine for something safer and more efficient. The idea of ionic liquids, especially those built on the imidazolium backbone, caught fire. 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate shows up in literature right around this time, a direct product of both necessity and creative experimentation rooted in physical chemistry and engineering. Before this, most looked at imidazolium salts mainly as curiosities. Through the turn of the millennium and into today's specialty chemical markets, dedicated process optimization propelled 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate into a workhorse in labs focused on green chemistry and recycling. At each stage, steady improvements in yield and purity emerged from both academic research and behind closed industrial doors. Having walked the halls of several universities, I watched the skepticism surrounding ionic liquids slowly pivot to practical acceptance as crucial components for energy storage and catalysis.
Product Overview
This chemical brings together an imidazolium cation—marked by an ethyl and a methyl group—and the hydrogen sulfate anion. The combination creates a salt that remains liquid at room temperature. What separates it from similar ionic liquids lies in its robust ionic character, high thermal endurance, and capacity to dissolve both organic and inorganic matter. The balance of acidic hydrogen sulfate and the flexible, organic-like imidazolium framework means it doesn't evaporate and can take the place of hazardous or flammable solvents. Commercial bottles, often appearing as faintly yellow viscous liquids, carry batch-specific data to confirm their unique ionic ratios and trace impurity levels.
Physical & Chemical Properties
In daily lab use, one notices its viscosity first—think honey more than water. It carries a slight odor reminiscent of sulfur and chalk. The compound rarely freezes under laboratory conditions, maintaining liquidity below zero Celsius and enduring thermal spikes almost to 300°C without decomposing. Conductivity stays high, a boon for battery and electrochemical cell development. Under an IR lamp or inside sealed flasks, the ionic bonds hold tightly, giving impressive chemical stability. Water and many common organics blend in with ease, showing the deep solvation range chemists rely on for catalysis, extraction, and electrodeposition. The hydrogen sulfate group offers mild to strong acidity, often outperforming many mineral acids as a proton source.
Technical Specifications & Labeling
Reputable suppliers publish tight specifications for purity, water content, chloride trace, and sometimes color. Typical purity standards run above 98%. Labels always highlight the hazard profile, with pictograms warning about environmental and health risks. Production scale—from 100-gram research packs to bulk multi-kilo drums—demands thorough quality assurance. Manufacturers pay close attention to the batch number traceability, date codes, and the presence of unique molecular fingerprints through NMR, IR, or elemental analysis. Customer safety and reproducibility rely on these technical guarantees holding up both in research and scaled-up syntheses.
Preparation Method
The standard route puts 1-methylimidazole in a controlled reactor, feeding in ethyl sulfate or ethyl hydrogen sulfate under an inert atmosphere. Operators mind the temperature and pH, adding extra steps if high water content threatens yield. Once the reaction runs its course, technicians strip out excess water under reduced pressure, often resorting to rotary evaporation or vacuum drying to squeeze out those last few weight percent. Filtration and repeated washing take care of residual starting materials. Properly prepared product streams through quality filter media, then gets dispensed in sealed, light-protective bottles to block UV degradation. It's a process that demands clean hands and thoughtful monitoring, because small errors snowball into lost product or impurity headaches down the line.
Chemical Reactions & Modifications
Lab hands reach for this chemical whenever a sharply selective acid catalyst is needed. Its proton-donating ability kicks off a long list of organic transformations, from esterifications to alkylations. The imidazolium ring stands up to most mild oxidants and reductants, meaning you can tailor reactions across a wide window without breakdown. Some creative chemists tinker with the side chains, producing closely related salts (swap the ethyl group, or plug in functionalized linkers) to tune solubility and activity. In extraction schemes, metal ions respond well to this ionic environment, forming complexes that can be filtered or stripped with minimal fuss. The chemical stability under a variety of pressures and temperatures lets process engineers build robust systems around it—working up acids, purifying pharmaceuticals, or recycling metals.
Synonyms & Product Names
Digging through catalogs or chemical registries, the compound shows up as [EMIM][HSO4] or 1-ethyl-3-methylimidazolium hydrogen sulfate. Some suppliers substitute “ethylmethylimidazolium hydrosulfate” or just “EMIM hydrogen sulfate.” The shorthand [EMIM][HSO4] gets frequent use in academic and patent literature. Cross-referencing with product identifiers in chemical inventories helps in verifying what lands on a benchtop matches regulatory filings and safety sheets—essential knowledge for compliance or troubleshooting.
Safety & Operational Standards
Anyone handling this material quickly learns respect for its irritant nature. Splash exposure threatens eyes and skin. The hydrogen sulfate’s acidity eats into gloves or lab coats with prolonged contact. Airborne droplets, though rare, can sting the respiratory system and raise long-term health questions. I’ve watched experienced staff preach strict glove, goggle, and lab coat routines. Fume hoods, splash shields, and spill kits stay on standby in labs that run kilo-scale reactions. Material safety data sheets carry guidance for first-aid steps, fire-fighting tactics, and long-term storage. Environmental disposal presents another hurdle—operators must collect spent liquid for authorized incineration or chemical waste treatment, never tipping it into ordinary drains. These habits anchor modern laboratory culture as much as fire drills and emergency eye-wash stations do.
Application Area
Electrochemistry research draws heavily from the unique properties of 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate. Battery groups use it as an electrolyte for fuel cells, supercapacitors, and specialized lithium-ion prototypes. Its thermal stability and ionic conductivity let devices store energy more efficiently, even under tough cycling or high current loads. In biomass conversion, green chemistry teams harness its acidity to break down cellulose, hemicellulose, and lignin—steps essential for turning agricultural waste into chemicals and fuels. Extraction specialists rig up liquid-liquid extraction systems to separate rare earths, pharmaceuticals, and even proteins, often skipping traditional organic solvents. Catalysis, especially acid-catalyzed carbon-carbon forming reactions, benefits from the long-lasting and selective action it provides. Coating and electrodeposition industries incorporate it both for surface treatment and for synthesizing thin films. New polymer synthesis and material modification processes count on its solvent power without flammability risks, cutting down both workplace hazards and insurance headaches.
Research & Development
Active research stretches across university and corporate labs worldwide. In every setting, workers look for new ways to drop costs, boost yield, and shrink environmental footprint. Many pursue tailored ionic liquids by changing the imidazolium ring structure, always comparing performance against 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate as a benchmark. Projects focus on recycling—how to recover and clean spent ionic liquids for reuse and how to strengthen their lifecycle sustainability. Others push into advanced catalysis: researchers validate applications in C–H activation, selective hydrogenations, and greener processes for dye and drug manufacturing. Quantum chemists and instrumentalists run simulations and NMR, IR, and X-ray experiments to unlock molecular-level insights. My own experience teaching graduate-level seminars showed that new data on these ionic liquids has a fast trip from theoretical models to wet-lab trials, with open sharing helping everyone climb the discovery ladder faster.
Toxicity Research
Toxicologists investigate both acute and chronic hazards linked to 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate. Studies flag moderate irritation for skin, eyes, and mucous membranes. Animal models reveal some risks with repeated high-level exposure, though far lower than old-school chlorinated solvents. Most teams point to the compound’s acidic nature as the main culprit behind tissue damage. Waste treatment researchers worry about aquatic impacts, since ionic liquids resist microbial breakdown. Experiments show low volatility, so inhalation risks stay down during normal lab routines—still, mass spills or fires could cause higher exposures. After years spent in chemical risk management meetings, I can say the consensus centers on proactive containment, specialized disposal, and regular training. Nothing replaces checking gloves and lab gear at every use.
Future Prospects
The outlook for 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate keeps stretching outward. Energy storage, carbon capture, and chemical recycling all demand stable solvents and smart catalysts, with this compound often at the center of the discussion. Startups and academic incubators bet that drop-in replacements for fossil-derived chemicals depend on ionic liquids’ tunable features. As regulations tighten on known toxics, the demand shifts toward high-performing, recyclable, and safer materials. Breakthroughs in bioprocessing and green extraction suggest wider adoption in pharmaceuticals, fine chemicals, and agroindustry soon. Costs, recycling hurdles, and questions about long-term ecosystem effects remain obstacles, but open research and transparent reporting smooth the way. Watching the momentum build, it feels clear that 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate’s story is only just getting started—less a solution in itself, more a versatile enabler for cleaner, smarter chemistry.
The Chemistry in Our Lives
Walking through a chemistry lab on a random Tuesday, you’ll spot all sorts of mysterious bottles and vials. Tucked on a shelf, you might find one holding 1-ethyl-3-methylimidazolium hydrogen sulfate. Most people have never heard of it outside of a lab, but those working with chemicals lean on this unique liquid all the time.
Helping Clean Up Industry: Solvent for Tough Jobs
Industrial chemists praise this compound mainly for its role as an ionic liquid. Unlike old-school solvents with strong smells and safety hazards, ionic liquids like this one have nearly zero vapor pressure, which keeps toxic fumes out of the workplace. In real-life numbers, companies that once worried about solvent emissions can now cut those emissions by as much as 95% using ionic liquids.
On the shop floor, this means safer air for the folks who stir the mixing tanks. Old solvents like toluene and acetone evaporate quickly, while 1-ethyl-3-methylimidazolium hydrogen sulfate stays put, giving time for more controlled reactions. This property becomes handy in making specialty chemicals, pharmaceuticals, or separating complex mixtures in a cleaner way.
Greener Catalysis for Modern Needs
The search for faster and cleaner chemical reactions never stops. Synthetic chemists rely on this ionic liquid as both a solvent and a catalyst. It takes on tasks such as breaking down cellulose to produce new biofuels. Switching to this route, researchers at places like the University of California found efficiency boosts that saved both money and materials.
By doing so, companies cut down waste from harsh acids and bases. The process works at lower temperatures, which keeps energy bills in check. Over time, this adds up to savings not just for industry but also for everyone downstream. Less power drawn from the grid means fewer emissions from coal or natural gas power plants.
Mimicking Water: A Niche Role in Electrochemistry
Lab techs use the salt for its special role in batteries, fuel cells, and supercapacitors. Electrochemical devices, which store or convert energy, need electrolytes that help move ions around without breaking down under stress. With its good conductivity and ability to stay stable at high voltages, 1-ethyl-3-methylimidazolium hydrogen sulfate fills that role. In the search for next-generation batteries—used in everything from electric cars to grid storage—the reliability of the electrolyte often makes or breaks the project.
Pushing toward Sustainable Extraction
In mining and rare-earth recovery, chemists try to get valuable metals without polluting water or soil. Ionic liquids like this one help separate metals, including platinum and gold, from ores that used to require harsh materials like cyanide. Switching to a less toxic process helps protect workers and surrounding communities.
Toward Broader Adoption
Costs and scale present the last big hurdles. As production methods improve, more companies look for ways to bring ionic liquids to larger markets. Trust in safety and efficiency, outlined in regulatory filings and peer-reviewed studies, helps win over industry leaders. Looking ahead, the ongoing push for greener, safer chemicals means compounds like 1-ethyl-3-methylimidazolium hydrogen sulfate won’t just stay in tiny vials—they’re already finding places in production lines and clean energy projects worldwide.
Understanding the Risks
I spent years in chemical labs, and every new compound brought a few moments of respect. 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate belongs to a class called ionic liquids. They grabbed everyone’s attention because they don’t evaporate easily, making them seem safer than traditional solvents. Still, labeling these liquids as “green” shouldn’t lead to carelessness. This compound doesn’t boil away, but it can make some nasty byproducts if it gets too hot or mixes with the wrong chemicals. More importantly, traces of hydrogen sulfate mean acid is in the mix, and acid doesn’t treat skin, lungs, or eyes kindly.
It’s important to read up on the specific dangers of both the imidazolium salts and acidic compounds in general. According to safety data sheets, contact with 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate can irritate skin and eyes. Breathing in its fumes, although less likely since it’s not very volatile, still brings risk—especially if it gets misted during handling. There’s always a chance for chemical burns, and those sneak up quicker than people expect.
Practical Safety Steps
The world of chemical safety relies on strong habits, not fancy equipment. The best labs use a few basic shields: gloves, goggles, and a real lab coat. I’ve seen people lose fingernails from acids far weaker than hydrogen sulfate, so skipping gloves just because you used this compound before never makes sense. Rubbing eyes mid-experiment without washing hands puts your vision at risk. Splash-proof safety goggles make a difference. Ventilation tops my list, since even low-odor chemicals in high concentrations can build up. An open window isn’t enough. Fume hoods carry vapors away before problems start, and everyone should know where the nearest eyewash and shower stations sit.
Spills should never invite improvisation. Acid-resistant pads and plenty of water for neutralization stand ready in well-prepared spaces. If something hits the floor or your clothes, deal with it right away. Ignoring a splash or thinking, “I’ll clean that up later,” never pays off. Even one slip can leave scars.
Why Respect Matters
Respecting chemicals isn’t about fear, but common sense. Universities and industrial sites who track injury reports show that most accidents happen because of complacency. Thinking a solvent “looks safe” or skipping protective gear for a quick experiment make up half the stories no one wants to tell. Even if 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate doesn’t release choking fumes, it can still burn skin, stain work surfaces, or interact with unsuspected impurities to create more toxic byproducts. Documented cases show that mixing with strong oxidizers or bases creates heat or new, dangerous compounds.
Choosing the Right Path
People who work with ionic liquids every day build safety into their routine. Regular training, easy-to-read labels, and good chemical organization lower risks for everyone. Those who rotate stocks, check for bottle leaks, and keep digital safety data handy avoid panic if spills or exposures happen. If any symptoms—redness, burning, trouble breathing—show up after handling, report it and get checked out. Fast action often turns emergencies into inconveniences.
1-Ethyl-3-Methylimidazolium Hydrogen Sulfate serves real purpose in research and industry, but like every strong tool, it demands respect. Making protection automatic takes less time than healing from a lab mishap. Chemistry builds its advances on this kind of practical trust: in the science and in the rules that keep science moving forward safely.
Seeing Beyond the Label
People working with chemicals like 1-ethyl-3-methylimidazolium hydrogen sulfate quickly learn that purity isn’t just a detail—it shapes every result in the lab or industry. This ionic liquid, often called EMIM HSO4, usually lands on shelves labeled “>99% purity.” That figure sounds simple enough, but there’s weight to those numbers. Researchers who pull product sheets know nothing frustrates project timelines more than finding unexpected traces of side products or water in a bottle that promises high purity.
Why Purity Drives Results
Having used EMIM HSO4 to dissolve cellulose and catalyze reactions, I know even trace metal ions or residual chloride from synthesis end up skewing experiments. When a supplier certifies “≥99%,” reputable labs back up that claim with HPLC, NMR, Karl Fischer titration for water, and ICP-MS for metals. Many also check melting point and visual clarity—even something as basic as a faintly yellow tinge can hint at issues. Moisture sits near zero for most research applications (<0.5%), since this liquid grabs water from air like a sponge. That’s why serious users triple seal bottles and store them in dry boxes.
The Details in Specifications
Specifications sheets pack the key numbers that define what buyers get: molecular weight (with EMIM HSO4, it’s 206.29 g/mol), density (about 1.32 g/cm3), and boiling or decomposition temperatures (usually the liquid stays stable below 220°C). These touchpoints tell engineers if a batch matches system requirements or needs pre-treatment.
Often, suppliers list chloride below 100 ppm, iron below 5 ppm, and sodium traces. Every number matters: run a supported catalyst with too much chloride, and the process fails; try to use low-purity EMIM HSO4 in electronics, and conductivity shifts ruin performance.
Industry Pressures and Solutions
Demand for ionic liquids grew in the last decade across biodiesel, solvents, and battery tech. With this, purity requirements keep tightening. In countries where regulation falls behind, knockoff or misrepresented batches can slip through. People in the chemicals business know this leads to real risks—equipment corrosion, toxicity, and environmental release.
Building confidence is a team effort. Labs should not only trust a certificate of analysis but confirm it in-house, especially for critical applications. Sometimes, purifying the ionic liquid further with activated carbon or passing it through ion-exchange resins helps. In my circles, some researchers always run a small pilot batch before full-scale reactions, just to make sure the reagent performs as expected.
The science behind EMIM HSO4 doesn’t stop with certificates. Regular, careful testing, partnerships with trusted suppliers, and staff who stay curious about what’s really in the bottle push the field forward. For those serious about reproducibility, good specs are the beginning, not the end.
A Closer Look at Responsible Handling
Most labs and facilities now work with ionic liquids like 1-ethyl-3-methylimidazolium hydrogen sulfate. This compound offers a lot of potential for chemistry, especially in catalysis and extraction. But, just like you wouldn’t toss bleach and vinegar together in the same cabinet, a smart approach to storage can mean better safety and longer shelf life.
Humidity Isn’t Just Weather Talk
Real-life experience shows that 1-ethyl-3-methylimidazolium hydrogen sulfate sucks up moisture from the air. One summer, I saw a sample left open for just a few hours and it went from fine powder to sticky mess. This material isn’t compatible with carelessness. Seal it up tight in airtight glass or PTFE containers. Desiccators make sense for anyone who wants the material to keep performing the way it should. Moisture affects how it works in reactions, and can change its physical properties. Any lab tech can tell you—replacing spoiled product costs more than dry storage in the long run.
The Right Spot in the Lab Matters
Sunlight, heat from radiators, and lab lights can break down even stable chemicals. I’ve seen students put sensitive chemicals on open shelving out of convenience, and the result was almost always a write-off. Tuck the container away from direct sunlight, ideally at room temperature. Refrigeration is unnecessary unless working in unusual environments, but no one wants heat speeding up unwanted reactions. If you store lots of chemicals, label each shelf by compatibility—acids, bases, solvents, ionic liquids. I created my own spreadsheet to track this and stopped playing chemical Tetris.
Containers: Cheap Isn’t Worth the Trouble
Plastic bags or low-quality jars seem easy, but ionic liquids attack some materials over time. Stick to glass or high-quality polymers. Screw tops with PTFE liners help prevent evaporation and drips. Make sure every label lists the chemical name, the opening date, and a hazard warning. Legible labels protect not only colleagues, but cleaners and students down the line.
Minimize Contamination Risks
I once watched a new hire scoop samples with whatever scooper was handy. Cross-contamination turned a $100 bottle into hazardous waste. Use dedicated, clean tools and never return leftovers to the original bottle. This stops degradation and extends chemical life. One mistake costs more time and worry than proper habits.
Safety Sheet Awareness Helps Everyone
Every bottle should come with a safety data sheet (SDS). Print these out or attach a QR code nearby so they’re always easy to check. These sheets list exactly what should be avoided—some metals, organics, and even some storage lubricants won’t mix safely with 1-ethyl-3-methylimidazolium hydrogen sulfate.
Thinking Ahead About Disposal
Nothing lasts forever. Once the chemical has reached the end of its useful life or the container’s compromised, don’t just dump it. Local regulations control disposal, and your environmental officer will have the right steps. Responsible disposal stops contamination of water sources and keeps the lab in good standing.
Smart Habits Bring Lasting Results
In my career, consistency beats shortcuts every time. Proper storage plays a real part in researcher wellbeing, experimental accuracy, and budget management. Respect for the quirks of substances like 1-ethyl-3-methylimidazolium hydrogen sulfate keeps doors open to new discoveries, and lets everyone work with confidence.
Real-World Use and Practical Insights
Handling 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate means dealing with the sort of ionic liquid that gets a lot of attention for its high thermal and chemical stability. People who work with solvents or green chemistry have probably seen it cropping up in syntheses, catalysis, or even biomass processing. Honestly, its popularity isn’t just hype. Stability matters when your workbench sits filled with sensitive, sometimes expensive reagents. Nobody wants their ionic liquid going bad before the job’s done.
Looking into its track record, this compound stands out because it doesn’t break down easily. Most imidazolium ionic liquids show resistance to both high temperatures and strong acids. I’ve seen research teams heat it up past 150°C with little decomposition, and yet, it keeps working as a solvent or catalyst. Hydrolysis isn’t much of a threat here, even under humid lab conditions. That means you can come back in a month and your sample's likely close to what you left—assuming you kept the bottle closed.
Risk Factors and Storage Habits
Chemists who store this ionic liquid in labs know that shelf life always connects to air and light. 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate prefers a dry and dark space. Leave the cap loose, oxygen will creep in, and the hydrogen sulfate part could pull in some moisture. Over time, absorption of water dilutes the original material and can reduce performance, but the liquid won’t suddenly turn dangerous or unusable. Labs aiming to keep reagent quality high use amber bottles and keep humidity low, sometimes adding silica gel in storage cabinets. Years of use show this habit pays off.
Oxidative processes don’t create problematic byproducts as quickly as in some other ionic liquids, but slow yellowing and a faint sulfur smell might mark an older batch. In ten years working with these, the only time I saw a total loss was from a loosely capped bottle left right next to a window in direct sun, which is about as bad as storage can get. Most bottles outlast their expiration label by a wide margin if storage rules get followed.
Support from Research
Several peer-reviewed studies back up what practitioners see on the bench. Thermal gravimetric analysis often shows negligible weight changes up to 200°C. Decomposition tends to kick in only with extended exposure to open flames or extreme heat, which doesn’t happen during regular synthesis. Spectroscopy results published by the Royal Society of Chemistry revealed that the imidazolium ring remains intact, even after repeated cycles of heating and cooling. Stability over a two-year testing period looked solid, with only slight variations in acidity and no new contaminants detected under sealed conditions.
Extending Shelf Life — Real Advice
Labs looking to improve shelf life rely on easy fixes. Sealing bottles carefully keeps moisture away. Using inert gas flushing, like nitrogen, helps further for bigger quantities. Labeling open dates and tracking color or odor changes gives early warning. Bulk buyers divide stocks into small bottles so only one faces repeated opening. Avoiding metal tools helps, since steel can sometimes catalyze slow reactions and nudge along degradation. Clean, plastic, or PTFE scoops prevent contamination and scratching. These daily habits make a noticeable difference on quality and save money.
Looking at Safer Chemistry
Mistakes happen, so it’s good that 1-Ethyl-3-Methylimidazolium Hydrogen Sulfate doesn’t form toxic gases in air or react with glass. Spills clean up without drama, and old or questionable batches rarely hazard safety, just reliability. Waste keeps low toxicity, which keeps disposal simple compared to volatile organics. As a result, more chemists look to use this ionic liquid as industry shifts toward greener, safer technology platforms.