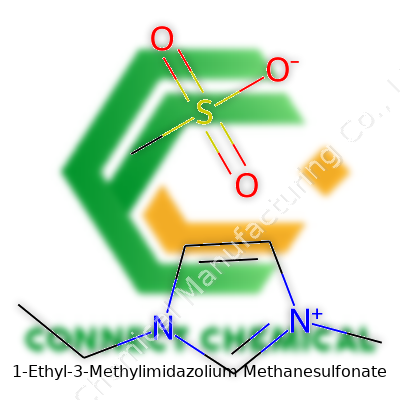
1-Ethyl-3-Methylimidazolium Methanesulfonate: Insight, Science, and Real-World Applications
Historical Development
Chemistry evolves with the demands of society, and 1-Ethyl-3-Methylimidazolium Methanesulfonate (EMIM-OMs) fits right into the story. Research on ionic liquids took off in the last two decades of the twentieth century, as industries sought safer, more energy-efficient alternatives to traditional organic solvents. The focus soon narrowed on imidazolium-based ionic liquids, including EMIM-OMs, thanks to their stability, conductivity, and sheer flexibility. In the lab, the switch to using compounds like EMIM-OMs sharply cut down volatile organic compound emissions, which mattered for anyone working in confined research spaces. Early skeptics asked if these “designer solvents” were more than just a fleeting trend, but demand for environmentally conscious chemistry has only grown stronger since then.
Product Overview
1-Ethyl-3-Methylimidazolium Methanesulfonate, often abbreviated as EMIM-OMs, belongs to the family of room-temperature ionic liquids. These salts, liquid at ambient conditions, open unique avenues between organic solvents and traditional electrolytes. Manufacturers often target ultra-high purity, aiming for clear, colorless to pale liquids with minimal impurities. My own early trials with EMIM-OMs highlighted its solvent capabilities, outperforming conventional choices in dissolving certain polymers and inorganic salts. This versatility bridges R&D laboratories and commercial pilot plants searching for solutions outside traditional chemistry playbooks.
Physical and Chemical Properties
EMIM-OMs stands out mainly due to its low melting point, often lower than 30°C, making it usable straight from the shelf without special handling. Its high thermal stability—tolerating up to 200°C or higher without breaking down—makes it robust in demanding applications. Unlike many volatile solvents, vapor pressure stays extremely low, so you smell almost nothing during use, and evaporative losses remain negligible. Its strongly ionic nature gives it a higher density and a faint viscosity, qualities that support its use as an electrolyte. Some researchers point to its remarkable solvating power for both organic molecules and metal complexes, reflecting a broad chemical compatibility profile that’s tough to match.
Technical Specifications & Labeling
Producers of EMIM-OMs typically list its purity at 99% or above, with residual water and halide content strictly limited. Containers typically come labeled with care instructions, hazard symbols, batch code, date of manufacture, MSDS access, and storage guidance (cool, dry, well-ventilated space). For chemists following strict protocols, technical data like conductivity (mS/cm), viscosity (cP), and decomposition temperature help match the product to research or processing needs. The labeling makes traceability routine, which becomes crucial for audits and safety reviews.
Preparation Method
Industrial synthesis of EMIM-OMs usually kicks off with the alkylation of methylimidazole using ethylating agents under controlled conditions to get the parent imidazolium salt. The reaction with methanesulfonic acid or its derivatives follows, producing the methanesulfonate salt. Researchers unfamiliar with ionic liquid preparation usually find the process very sensitive to water and by-products, requiring rigorous purification—often through repeated washing, filtration, vacuum drying, and chromatography. Every step can leave trace contaminants that change the physical behavior of the final product. In my experience, patience during washes is rewarded when high-purity ionic liquids behave reliably in both analytical and preparative chemistry.
Chemical Reactions & Modifications
EMIM-OMs doesn't just work as a solvent, it readily participates in chemical transformations. Strong acids or bases cleave the imidazolium ring, though under practical conditions, EMIM-OMs usually holds strong. Chemical engineers searching for tunable properties sometimes look at swapping methanesulfonate for related anions through metathesis. This anion exchange lets chemists swap out dissolution profiles or hydrophilicity to fit new tasks. I’ve seen teams push the envelope by grafting functional groups onto the imidazolium ring, tuning performance in catalysis and separation science.
Synonyms & Product Names
You might spot other names for this compound, sometimes as 1-ethyl-3-methylimidazolium methylsulfonate or EMIM Methanesulfonate. Suppliers sometimes use product codes like EMIM-OMs or EMIM-MeSO3. For record-keeping, the CAS number offers clarity and avoids mix-ups, as the world of ionic liquids hosts a dizzying range of similar-sounding compounds.
Safety & Operational Standards
Working safely with EMIM-OMs always starts with basic precautions: gloves, goggles, and lab coats. Safety Data Sheets stress that, though less volatile, accidental spills call for prompt cleanup due to moderate toxicity and possible skin or eye irritation. Chronic exposure hasn’t shown up as a major risk in published studies to date, but prudence in handling any modern solvent remains vital. Storage in tightly closed containers reduces risk of accidental moisture absorption and cross-contamination. In a practical sense, these standards keep both seasoned and new chemists protected in fast-paced lab settings.
Application Area
Applications keep expanding every year. Battery and supercapacitor engineers put EMIM-OMs to work as electrolytes, counting on its high ionic conductivity and thermal stability. Green chemists swap out traditional, smog-producing solvents in organic synthesis or extraction with ionic liquids like EMIM-OMs, aiming to simplify both downstream processing and waste management. Enzyme-catalyzed reactions run in this medium benefit from improved stability and higher yield in certain biotransformations. Industrial-scale gas capture projects, including those targeting carbon dioxide, use EMIM-OMs for gas absorption and separation. In my own experience, its performance in cellulose processing stands out—dissolving biomass with far less mess and energy than older solvents.
Research & Development
Innovation around EMIM-OMs comes from both curiosity-driven and industry-sponsored research. Academics dig into the molecular reasons behind its solvation properties, reporting on how subtle changes in temperature, impurities, or water alter function. Industrial chemists tweak formulations to bring costs down or boost recyclability, responding to sustainability regulators. I’ve followed research on using EMIM-OMs in metal recovery, especially from e-waste streams, where the hope is to strip value from discarded electronics without harsh acids or high temperatures. Multi-disciplinary teams keep finding new angles—from pharmaceuticals to polymers to clean energy storage—reflecting a healthy pipeline of research papers and patents.
Toxicity Research
Studies up to now have shown that EMIM-OMs, like many modern solvents, carries moderate toxicity if mishandled, but doesn’t rank among the most hazardous lab chemicals. Acute exposure studies on animals often report mild or moderate effects at realistic concentrations. Bioaccumulation doesn't seem to raise alarms, but regulators pay close attention, especially with new molecules that might enter wastewater or food chains. Long-term data remain limited, so routine assessment and stewardship are essential, both in the lab and at production sites. Every chemist knows the tale of a “harmless” compound later reclassified as hazardous—ongoing vigilance stays part of the routine with any new material.
Future Prospects
The road ahead for EMIM-OMs looks active. Energy storage and transfer companies keep searching for tougher, safer electrolytes, while large chemical processors search for greener, recyclable mediums to cut both costs and emissions. If the cost of synthesis drops and recyclability gets even better, its adoption will keep growing. Regulations will shape this future, pushing more data on health and environment, while real-world field results either confirm the hype or steer developers in new directions. My own experience suggests future breakthroughs may arrive with hybrid systems—ionic liquids paired with novel nanoparticles or enzymes—creating solutions that push chemistry forward while aiming for safer human and environmental outcomes.
Getting to Know This Ionic Liquid
1-Ethyl-3-Methylimidazolium Methanesulfonate, with its mouthful of a name, pops up often in labs and industry workbenches. Chemists call it an "ionic liquid," which means it’s a salt that stays liquid even at room temperature. That’s not all that common. Most salts want to be solid, think table salt. So, a runny salt grabs people's attention—especially folks trying to solve problems around dissolving tricky materials and running efficient processes without the mess of volatile solvents.
Applications in Electrochemistry
Electrochemistry laboratories have been some of the earliest adopters. This liquid salt gives batteries and supercapacitors a nudge by letting ions move freely—important for charging and discharging. I’ve talked to researchers who switched from old-school organic solvents to this stuff and cut down on fire risks. It doesn’t puff out dangerous fumes, and that matters when you spend hours in a closed lab. The high ionic conductivity means more bang for your buck, too: ions move faster and hit the electrodes just right, often improving overall energy storage.
Solvent for Green Chemistry
Traditional solvents—like acetone or toluene—cause headaches for workers and the planet. 1-Ethyl-3-Methylimidazolium Methanesulfonate flips that story. It doesn’t evaporate easily under regular room conditions, so air quality improves, and chemical exposure drops. Fact: many universities have switched to this type of solvent to lessen their hazardous waste. It works well for reactions where water or old solvents just can’t keep up, like dissolving cellulose from plants. Processing biomass without choking the air with noxious fumes turns out to be a big win, both for worker safety and sustainability.
Catalysis and Material Science
Lab folks looking to speed up reactions often grab this ionic liquid for its stability and knack for dissolving both salts and organic compounds. Instead of settling for clumpy mixtures or outright failures, reactions run smoother. Some colleagues in pharmaceutical research tell me they boost yields in certain drug-making steps while using less energy and cutting out toxic byproducts. Engineering students who tinker with advanced polymers use the same liquid because it helps shape and customize new materials with fewer sticking points.
Carbon Capture and Environmental Tech
Many scientists worry about the toll of climate change. I’ve seen reports where this ionic liquid caught the attention of CO2 capture teams. Its strong solubility means it grabs carbon dioxide from air or exhaust streams and hangs on tightly. That’s helped push pilot projects in power plants aiming to trap and recycle carbon emissions. While the tech isn’t everywhere yet, early results look promising thanks in part to this unique compound.
What’s Next for This Liquid?
The chemical sounds niche, but it’s finding more homes every year. Lab workers who focus on greener methods keep exploring new reactions or extraction tricks. Battery designers who chase longer-lasting and safer storage bank on its thermal and electrochemical stability. Across these fields, people notice less harshness and more efficient outcomes, which matter for health, climate, and bottom lines.
The Meaning Behind Chemical Purity
Every bottle or bag of laboratory chemicals shows off a purity percentage, and that number carries real weight. At its simplest, purity means how much of the contents is the chemical you’re after versus everything else. For those working in quality control or research, spotty purity can knock results off course. I remember testing reagents in school and seeing strange readings because a “pure” product snuck in just enough impurity to throw everything into question. Even a chemistry hobbyist can get frustrated by unreliable products.
Grades Tell a Story
Producers sort their products by grade: technical, laboratory, reagent, analytical, and on through to pharmaceutical. Each grade matches up with certain tolerances. Analytical grade, the good stuff for sensitive experiments, puts impurity levels under a magnifying glass. Something labeled “technical” works fine in manufacturing but could bring disaster in drug development. The U.S. Pharmacopeia (USP), American Chemical Society (ACS), and other organizations set clear benchmarks so there’s less guesswork in picking chemicals for any project.
How Purity Impacts Outcome
Pilot projects and full-on factory processes can both spiral from impurities. A paint chemist chasing vibrant, long-lasting color jobs needs high-purity pigments to keep shades consistent. In food production, residue from cleaning or cross-contamination can risk not just quality, but safety. Over in the electronics world, chips fail if rogue atoms get mixed in the wrong place. One example that sticks out is with labs prepping batches for students—one off-spec bottle leads to inaccurate results, wasted time, and plenty of frustration.
Testing Methods and Certification
Manufacturers use a range of tests: titration, chromatography, spectrometry, and others. These methods check for known troublemakers and measure trace contaminants to the part-per-million. They don’t just print “pure” on a label—labs issue a certificate of analysis (COA) to back it up. Reputations ride on these details matching up with what’s inside the package. Reading a COA has become a habit for anyone serious in the field, just the same way cooks check expiry dates. If a supplier doesn’t pony up a recent certificate, it ought to raise an eyebrow.
Common Mistakes and Better Practices
One mix-up comes from not matching a chemical’s grade to its purpose. A warehouse may grab industrial bleach for cleaning, but someone doing research needs lab-grade sodium hypochlorite, not something loaded with stabilizers. Lawmakers and public health agencies lay down strict boundaries for chemicals in medicine or food. Still, errors happen when shortcuts tempt rushed buyers or untrained staff. Clear labeling and basic training go far. I’ve seen busy teams avoid huge headaches just by asking suppliers to explain their grades upfront.
Moving Toward More Accountability
Calls for transparency keep echoing. Customers now ask detailed questions, and reliable suppliers update them quickly. Tech advances have made quality checks more affordable, so real-time tracking for purity isn’t a pipe dream anymore. Those who value accuracy in their work—whether a DIY scientist or a global manufacturer—find themselves sharpening their focus on source information and supplier reputation. It’s not about perfection; it’s about not being left in the dark.
Why Practical Storage Really Counts
Storing chemicals is stressful for anyone who’s spent hours in the lab. I’ve seen bottles leak, labels peel, and once, a shelf collapse. The stakes climb with specialty chemicals like 1-Ethyl-3-Methylimidazolium Methanesulfonate. This ionic liquid gets praise in research circles for dissolving cellulose and pulling off clever tricks in electrochemistry. Forgetting safe storage brings a world of headaches—from lost investments to actual injuries.
Real Concerns, Real Experiences
This compound can pull moisture from the air. Pulling the stopper in a humid room feels harmless, but anyone who's opened an old bottle, only to find a sticky mess, knows just how quickly careless storage wastes material. Keeping it truly dry isn’t just bureaucracy; it keeps properties right and labmates safe.
Temperature swings spell trouble. I remember pulling a bottle out of an overcrowded cabinet, only to find it sticky and slightly discolored. Small mistakes like stashing it near heat sources or in sunlight will warp quality, knock off purity, and possibly trigger a mess that could have been avoided with a little more care.
The Big Three: Container, Atmosphere, Temperature
Reliable glass, with a sturdy seal, outperforms plastics. Some ionic liquids sneak through cheap polymers and corrode metal caps. Investing in tight glass stoppers or Teflon-lined lids is worth every penny—no one wants to mop up a chemical that tears through seals. I’ve opened plastic bottles that felt slightly soft from slow leaching, so erring on the side of caution saves headaches down the line.
After years of working with these liquids, I learned desiccators aren’t just for fancy crystals. Throwing in a scoop of fresh desiccant and keeping them away from damp corners goes a long way to preserve material. Silica gel or phosphorous pentoxide work well, and it’s always smart to rotate and inspect for color change—dry doesn’t stay dry forever in a busy lab.
Ambient room temperature works as long as the area stays away from radiators, ovens, and direct sun. Above 30°C, degradation creeps in. In the winter, some labs get cold, but freezing isn’t the problem; it’s the big temperature swings and repeat cycles of hot and cold that slowly damage the compound.
Label Clearly, Check Regularly
Every bottle gets a clear date and contents. Faded marker or missing tape lets confusion into the lab. I’ve seen strangers open mystery bottles, hoping for methanol and getting a much nastier surprise. Regular checks for leaks, discoloration, or crystals are good habits. These simple steps catch problems before they become real hazards.
Training and Accountability
Nobody’s born knowing proper storage. Training new lab members pays off. It might take one lunch break to go over what goes where and why details matter, but that saves weeks of confusion and waste. In safe labs, everyone takes responsibility for daily checks. Treating chemical storage as routine, not as an afterthought, builds a culture where everyone looks out for quality and safety.
Building Trust through Small Actions
Meeting regulatory guidelines isn’t a finish line; it’s a minimum. Each good habit, from using glass to keeping a dry box stocked and logging dates, builds trust in the data and safety of the whole team. Solid storage practice isn’t glamorous, but it keeps research on track and people healthy. Real safety starts in the daily details.
Looking Beyond a Product Label
Every day, products with warning labels land in homes, schools, and workplaces. Even after reading labels, the risk sometimes feels confusing. Many folks assume products getting sold on shelves means they're completely safe, but history has proven that's not always true. Decades ago, people trusted asbestos insulation in homes. Stories from families facing illness later show how assumptions can backfire. Being careful with new chemicals or gadgets often protects your health, family, and neighborhood.
The Challenges of Safe Handling
New gadgets and chemicals can sneak into daily life, changing routines. Workers at shipping warehouses, science class students, and DIY homeowners all run across batteries, sprays, cleaners, plastics, and solvents. Each holds a different set of dangers. Lithium batteries power everything, but stories about fires started in garages tell us they're not harmless. Drain cleaners can burn skin fast if handled without gloves, and their fumes make it tough to breathe. Farmers applying pesticides outside need to think about wind and where that drift lands, not only on crops but possibly on skin. Precautions sound repetitive—gloves, goggles, and good ventilation, but ignoring those steps causes accidents nobody wants to face.
Trust and Sourcing Information
Learning which products carry risks isn't always clear-cut. Marketing sometimes hides warnings in fine print or behind fancy promises. It takes a mix of common sense and seeking solid information. Government agencies like OSHA and the EPA list guidelines and incidents. In my work, checking the Safety Data Sheet (SDS) brings up surprises. What seems harmless—like glue or art supplies for kids—sometimes reveals hidden respiratory risks or allergy triggers buried in technical language. Relying only on store packaging rarely helps, so searching out these resources protects everyone from unplanned emergencies.
Why Experience Taught Us Caution
Many have memories of minor cuts or burns that felt like accidents at the time but turned more serious after learning the product’s real dangers. A close colleague once ended up in the hospital after mixing cleaning products at home, not realizing that ammonia and bleach react, making toxic gas. Those moments put the entire issue in perspective: products marketed for ease can cross the line to hazardous before users realize what’s wrong.
Protecting Homes and Workplaces
Care comes from preparation. For anything new, read what the manufacturer says, not just glossy ads. Use gloves and masks for harsh chemicals, even if the job seems small. Store containers out of reach of children and pets. Batteries and oils belong far from heat or direct sun. Pace out work spaces so spills or fires won’t endanger the whole room. Check for warning symbols and always look for first aid guidelines. Regular training or brief safety reviews in workplaces keep people alert and ready to handle routine goods that, under the wrong conditions, cause real trouble.
What Works as a Solution
Staying updated pays off. Local fire stations and waste services offer talks about safe disposal, storage, and transport—especially for things like paint thinners or old electronics. Workers should push for clear procedures, not rely on “that’s how it’s always been done.” Clear labeling, education, and responsible habits keep both seasoned professionals and young learners away from danger. Risks come with modern life, but clear thinking, demand for information, and community dialogue push incidents down and safety up.
The Backbone of an Ionic Liquid
1-Ethyl-3-methylimidazolium methanesulfonate stands out in the family of ionic liquids. Chemists and engineers who’ve worked with solvents or alternative electrolytes have bumped into this name. Its chemical formula: C7H14N2O3S. The molecular weight lines up at 206.26 g/mol. Simple numbers on paper, but they carry weight in labs, factories, and even university classrooms.
Why the Structure Matters so Much
It’s more than just counting atoms. The formula carries a message about stability and the roles this salt plays in green chemistry. Many look at the imidazolium ring as being the game-changing secret. The 1-ethyl-3-methyl- part refers to the pattern of carbon side chains attached to the nitrogen atoms in imidazole. That means changes to these side groups often shift key properties—melting point, viscosity, how it interacts with water, and even safety profile. Most folks want these details nailed down before stacking bottles on a shelf.
Methanesulfonate Brings a Unique Edge
Pairing the imidazolium cation with a methanesulfonate anion shapes its use cases. Many common ionic liquids use halides or tetrafluoroborate anions, but methanesulfonate brings lower toxicity and helps with biodegradability. I found this out during a solvent screening experiment where environmental impact mattered. The anion helped the salt resist breaking down under heat and light, which allowed repeated use in different reaction cycles. This reduces waste, cuts cost, and lessens regulatory headaches.
Real-World Roles Beyond the Lab Bench
This salt mixes science with engineering. I’ve seen it serve as a solvent for cellulose—a polymer that usually shrugs off anything short of pretty aggressive reagents. In battery projects, the same salt boosts ionic conductivity while keeping thermal runaway risks at bay. There’s talk about its use in CO2 capture and as a green reaction medium for catalysis. All this depends on a reliable knowledge of its stoichiometry, which keeps supply chains and safety protocols tight.
Addressing the Challenges
Working with ionic liquids reveals plenty of surprises. Listen to someone ordering a drum: they’ll worry about purity, consistency, and whether a supplier slips in water or even chloride ions. The community has responded by developing tighter QC checks—think NMR and Halide analysis. Best practice also calls for storage under dry, airtight conditions, not left in a corner hoodie pocket or near open windows. By paying attention to these details, folks can avoid headaches with drift in results and product instability.
Looking Forward: Getting More from Each Molecule
Interest in these salts continues to grow. Researchers are tailoring the imidazolium backbone and side chains, seeking customized properties for niche industrial processes. Sustainability teams want ionic liquids with the lowest environmental footprint. By knowing basics like the formula—C7H14N2O3S—and how each atom shapes performance, it’s possible to design better systems for the future. Exploring these details isn’t just academic; it gives workers and innovators an edge with safety, efficiency, and responsibility in mind.