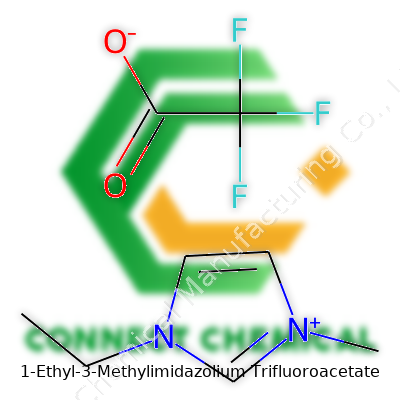
1-Ethyl-3-Methylimidazolium Trifluoroacetate: A Deep Dive
Historical Development
The story of 1-Ethyl-3-Methylimidazolium Trifluoroacetate traces back to a period when the race for greener, more efficient solvents grew urgent. Chemists kept reaching for alternatives to volatile organic compounds. This search turned, over several decades, toward “ionic liquids,” which don’t evaporate at room temperature. Researchers at the start played with different cations and anions, realizing soon that imidazolium-based salts stood out for their stability and versatility. As more teams began sharing successes in cellulose processing or catalysis using such salts, every new discovery seemed to open up fresh branches, leading right to materials like 1-ethyl-3-methylimidazolium trifluoroacetate. It’s no exaggeration to say these liquids have become a symbol of chemistry’s changing priorities—pushing beyond the old rules of solvents and seeking to shrink environmental footprints.
Product Overview
1-Ethyl-3-Methylimidazolium Trifluoroacetate ranks among the ionic liquids that many labs favor when traditional organic solvents just don’t do the trick. Its structure—a cation built from an imidazolium ring, ethyl and methyl groups, paired with a trifluoroacetate anion—delivers the right mix of low volatility, chemical stability, and ionic conductivity. What stands out is its ability to dissolve tricky cellulose and its good behavior with biopolymers. It isn’t some obscure specialty chemical: researchers from biorefining labs to electrochemical engineers trust this salt for both experimental and practical processes, from separating biomolecules to running batteries or fuel cells.
Physical & Chemical Properties
A clear or pale straw-colored liquid, this ionic liquid refuses to evaporate under normal lab conditions, which makes it fundamentally safer than many volatile organics. Its melting point sits well below room temperature. Viscosity typically hovers near that of heavy oils, but adding water drops it substantially, which practical chemists exploit when scaling up a project. Its density stays higher than water’s, a boon in layering or extraction. As a trifluoroacetate salt, its thermal stability also surprises: the compound resists breaking down, tolerating the temperatures needed for key syntheses. One property often noticed: its excellent ionic conductivity. That trait has driven some creative uses, especially among electrochemists looking for stable, conductive media in sensitive measurements.
Technical Specifications & Labeling
Synthesized at high purity, this imidazolium ionic liquid usually carries a CAS number of 145022-44-2 and a formula of C8H11F3N2O2. Purity checks need to confirm minimal halide and moisture content because even tiny impurities can complicate reactions—especially for those working on biomaterials. Labels always reveal more than just name or formula; information about batch, water content (Karl Fischer results), and residual acid content gives buyers confidence they’re getting what’s promised. Packaging typically relies on amber glass, since light and humidity can slowly cause decomposition and alter its properties. Safety data, down to appropriate response in case of skin contact or inhalation, is essential right on the label, respecting global chemical standards.
Preparation Method
Preparation starts from 1-ethyl-3-methylimidazolium chloride or bromide. Reacting that with silver trifluoroacetate in a solvent like water or acetonitrile causes an ion exchange, letting researchers filter out silver halide byproduct. Once the reaction’s complete, purification depends on removal of any leftover silver, halide, or water—often under reduced pressure and sometimes under dry nitrogen. In daily practice, this means several steps of filtering, rotary evaporation, and vacuum drying, leading to a near-waterless final product. Chemists doing this routinely worry most about trace water or acid altering its reaction profile down the line. Rigorous quality control has become an accepted part of manufacturing, balancing cost and purity.
Chemical Reactions & Modifications
1-Ethyl-3-Methylimidazolium Trifluoroacetate often acts as both solvent and reactant. In biomass work, the salt grabs onto cellulose chains, breaking their crystalline structures and making the notoriously insoluble polymer processable. Thanks to its weakly basic anion and stable cation, it rarely interferes in catalytic cycles—this earns it favor in organic transformations involving transition metals or even enzyme-catalyzed steps. At the lab bench, I’ve seen it used to modify surfaces, such as in ion-exchange resins, and to dissolve stubborn polysaccharides, producing derivatives impossible to access using standard solvents. Some researchers have tested anion exchanges, swapping trifluoroacetate for more reactive groups or structuring blends of ionic liquids for hybrid properties. Not everything works: certain strong acids or strong nucleophiles can degrade the cation, so reaction design calls for forethought.
Synonyms & Product Names
Anyone searching the catalogues will find this ionic liquid as EMIM TFA, 1-ethyl-3-methylimidazolium trifluoroacetate, or just [EMIM][TFA]. Some trade names simplify to EMIM-TFA or EMMI-trifluoroacetate, depending on the provider. Each name links to the same core product, but careful attention saves confusion since closely named salts with different anions, like chloride or acetate, behave quite differently in sensitive applications. The CAS number often serves as the final check among researchers and procurement teams alike.
Safety & Operational Standards
Handling this ionic liquid keeps safety routines on high alert. Splash risks to eyes or prolonged skin exposure aren’t trivial; its low volatility doesn’t mean it’s harmless. Labs install local extraction or run under closed systems for good reason. Many teams lock containers away from light and moisture, since either can chip away at stability. Disposal shows the real challenge: as with other fluorinated compounds, break-down products can stick around in the environment. That’s pushed many toward tight waste management and, where possible, recycling or recovery. Training new staff about spill kits, eye protection, and exact storage conditions comes before the first gram leaves the cabinet.
Application Area
Its flexibility touches a pile of industries and academic projects. Paper and textile engineers have tried using this liquid for dissolving cellulose, spinning it back out as novel fibers or films. Electrochemists have written reports about its use in supercapacitors and lithium cells, praising the conductivity and stability in demanding settings. Chemical engineers running biomass fractionation or seeking to isolate lignin have gravitated toward this salt for its ability to unlock new routes for conversion. Biochemists playing with enzyme or protein solubilization have coaxed new activity from reluctant biocatalysts with help from EMIM TFA. In my own experience, trying to get stubborn lignocellulose to dissolve, almost nothing matches the push this ionic liquid provides.
Research & Development
Research has reached a fever pitch, with new papers hitting journals every quarter. Teams around the globe compete to tweak cation and anion pairs, hoping to fine-tune salt properties for new separation or catalysis approaches. Much of this work converges on sustainability claims—cutting down on toxic waste, capturing previously unusable biopolymers, or improving the life cycles of batteries. Researchers continue probing how this compound interacts with different microbes and enzymes, offering new doors for green chemistry. Upstream, manufacturers scale up trials, using continuous reactors and near-infrared sensors to shave impurities from every kilo. Collaboration between academic labs and scale-up engineers leads straight into the next round of breakthroughs; you can feel the momentum each year as new abstracts roll out at chemical society meetings.
Toxicity Research
Toxicology always carries surprises, and this salt is no different. Tests in model plants or algae highlight potential persistence concerns with the trifluoroacetate anion, since fluorinated chemicals don’t degrade easily. Cell studies show low acute toxicity in many mammalian lines, giving hope for safe handling, but chronic effects haven’t been fully charted. In practice, accidental release—whether down a drain or into soil—raises flags. The lack of evaporation means spills hang around, giving chemists plenty of time to mop up, but also keeping environmental fate in question. Industry and academia call for more long-term ecotoxicity data before approving very large-scale uses. In the meantime, waste management and spill response protocols set a high standard for everyone handling the material.
Future Prospects
Looking ahead, the uses for 1-ethyl-3-methylimidazolium trifluoroacetate continue spreading out, especially as industries keep searching for alternatives to toxic or volatile solvents. Biomass fractionation and recovery stay at the forefront, with new patents for wood pulping, paper production, or textile spinning. Electrochemical systems—especially for grid-scale storage—may bank on this salt’s ability to stand up under load. Sustainability efforts likely shape every step of the way; more companies want to prove the full life cycle of these ionic liquids closes the loop, from sourcing feedstocks right through to safe destruction or recycling. What’s certain is that the field refuses to stand still. With every round of process optimization and mechanistic insight, the chemistry community moves one step closer to the kind of renewable, safe, and efficient solutions that seemed far off just a decade ago.
The Real Shape of an Ionic Liquid
1-Ethyl-3-methylimidazolium trifluoroacetate catches the eye in chemical circles, especially among folks working with ionic liquids. This isn't some fancy jargon for a new cleaning product. This type of molecule holds the power to change how researchers clean up old processes, improve batteries, or work with tricky-to-dissolve substances. Most of the value comes from how its atoms line up and interact, not just a bullet-point list of its possible uses.
Breaking Down the Parts
The name gives away the blueprints. At the core sits 1-ethyl-3-methylimidazolium, a charged ring made up of three carbon atoms and two nitrogens. In chemistry labs, the ring structure always means business since it brings stability and a knack for holding onto energy. One edge wears an ethyl group—a short, two-carbon tail. Another side sports a methyl group, which only adds one carbon. Stick these groups on the imidazole ring, charge the whole thing positively, and you've got the cation side of this molecule.
Add in trifluoroacetate: this is the counterion, with three fluorine atoms clinging to a carbon that’s also double-bonded to an oxygen, then hooked to a carboxyl group. Because trifluoroacetate loves electrons, and the cation wants to shed its charge, they balance each other out.
Why the Shape Matters
The cation and anion never fit together as tightly as old-school salts like table salt. That loose grip means the compound stays liquid well below the boiling point of water. Chemists pay attention to how tightly ions hug each other, because it affects how well they dissolve other stuff or transfer electric charge. I’ve handled ionic liquids like this in the lab and noticed they flow more like syrup than anything sharp like acetone.
This unique structure gives 1-ethyl-3-methylimidazolium trifluoroacetate a reputation for low volatility and strong solvent abilities. In plain English: You can mix it with things that most regular solvents can’t touch. I’ve seen colleagues use it to dissolve cellulose from plant matter. Try doing that with ethanol or water, and you’ll wait longer than your patience can carry you.
Applications Rely on Structure
The way 1-ethyl-3-methylimidazolium lines up with trifluoroacetate ions lets it play nice with a wide range of chemicals. Battery engineers use its forgiving structure to move ions inside advanced batteries. In our lab we’ve tested its compatibility in green chemistry applications—where it replaces nastier solvents—because it rarely evaporates into the air or leaves unwanted residues.
The fluorine atoms sitting on the trifluoroacetate portion also ramp up chemical resistance. Where a less sturdy ion might snap under strong acids or bases, this one keeps its cool, which means it sticks around for a wider range of chemical reactions. That chemical backbone lets researchers tackle reactions at higher temperatures and with tougher substrates.
Pushing for Safer Use and Environmental Insight
Big breakthroughs often bring new challenges. Handling fluorinated compounds calls for extra care because they stick around in the environment once released. Some scientists now look for versions with less persistent anions but want to keep the unique benefits this structure offers. Waste treatment and recycling strategies for ionic liquids like this one deserve investment, as does research into safer alternatives or tweaks to the existing molecule that lower its environmental footprint.
Understanding the intricate chemical structure of 1-ethyl-3-methylimidazolium trifluoroacetate sharpens the toolbox for scientists, and opens up cleaner technologies. Attention to molecular detail has never felt more urgent as researchers seek smarter solutions for old problems.
Getting to Know the Compound
1-Ethyl-3-methylimidazolium trifluoroacetate, often referred to as [EMIM][TFA], has built up quite a reputation in the last decade for practical reasons. Speaking as someone who has seen academic labs cycle through countless solvents and ionic liquids, I noticed this one standing out. Its structure balances stability and performance, letting chemists and engineers push the envelope with tricky tasks that other liquids just muddle through. The acetate part of the molecule offers a particular edge, which speaks volumes for tricky tasks in chemistry.
Sustainable Biomass Processing
Green chemistry circles talk a lot about sustainability, but actually turning tough materials like wood or agricultural waste into fuel or valuable chemicals doesn’t happen without the right solvent. In many labs, [EMIM][TFA] steps up to break down cellulose and hemicellulose, letting enzymes reach the sugars hidden inside. Without this step, efforts to create truly renewable fuels run into dead ends. Take a look at a biofuel pilot plant, and there’s a good chance you’ll see this compound unlocking new molecules from what used to be leftovers. Studies in journals like Green Chemistry and Biomacromolecules highlight how it tackles this challenge where cheaper solvents just fail. This is more than minor progress—cheaper biofuels matter for rural economies and climate change alike.
Enzyme Chemistry and Industrial Biocatalysis
Scientific progress in enzyme engineering depends on controlling the environment around proteins. [EMIM][TFA] has made a name for itself by helping enzymes work outside their comfort zones. For instance, lipases and cellulases, both essential for pharma and renewable energy, manage higher activity in this ionic liquid. This opens up ways to make drug ingredients faster or convert plant waste. From my experience with colleagues at university research groups, enzyme reactions easily stall out or get contaminated in water-heavy solutions, while reactions in [EMIM][TFA] tend to stay clean and productive.
Industrial Separation and Extraction
Traditional processes for separating chemicals rely on old-school organic solvents that bring along safety risks and environmental headaches. [EMIM][TFA] cleans up that picture. Refineries and chemical factories use this ionic liquid to extract things like rare earth elements or target products from fermented mixtures. There’s a sharp focus now on rare earth recycling, mostly because electronics demand is surging. This solvent’s design fits the job, since it stabilizes target molecules and ignores much of the gunk. Reports show that yields and purity rival traditional toxic solvents, but with a safer and less flammable approach. Having seen failed extractions ruin weeks of work, I see why engineers welcome a tool that gets the job done without so much cleanup.
Carbon Capture and Environmental Solutions
The world grapples with cutting carbon emissions, and chemical scrubbing stands on the front line of that struggle. A team in the UK demonstrated that [EMIM][TFA] soaks up carbon dioxide at rates that stand out compared to older liquid amine technologies. Businesses chasing zero-carbon goals look for solutions that last, don’t degrade under tough conditions, and work across temperature swings. This ionic liquid checks the boxes. Industrial testbeds have ramped up trials with this solvent in actual CO2 removal stacks, seeing drop-in improvements without risky side reactions. From my own networking at climate technology meetups, companies always ask about chemicals that can win the struggle against corrosion and loss of efficiency—[EMIM][TFA] has sparked more than one hopeful conversation.
Room for Growth Managed by Safety
Safety and price put real limits on which chemicals take hold in industry. With [EMIM][TFA], careful handlings, such as using gloves and fume hoods, go without saying. Its synthesis costs land higher than those for runoff-the-mill solvents, but as production scales and recycled streams mature, the numbers improve. Vetting by academic and industrial teams for toxicity and long-term impact continues; this attention sets up a future where practical use balances performance with responsibility.
What Purity Really Means
Asking about the purity of a product does more than just scratch the surface. In many industries, including chemicals, pharmaceuticals, and food processing, purity directly tracks back to quality and safety. A percentage stamped on a certificate of analysis—98%, 99.9%—tells a story about just how much of the final product comes from the expected material and how little comes from unwanted by-products or contaminants.
This difference matters. Slight impurities in a batch of pharmaceutical ingredients can alter the way a medication works in the body, or set off allergic reactions never seen in clinical trials. From my experience working alongside lab technicians, even a slip in standards, unnoticed at first, can become a recall months later. The U.S. Food and Drug Administration and comparable agencies abroad set strict limits for a reason. These limits aren’t just boxes to check. They protect people who might otherwise pay the price for shortcuts.
Specifications: Not Just the Numbers
Specification is another layer. A full specification sheet doesn’t stop at purity. It covers melting points, moisture, particle size, residual solvents, and even color. Each test ties expectations to reality—good suppliers don’t hide these numbers or wait for someone to ask. Instead, they put them front and center, open to scrutiny.
A few years ago, a friend ran into trouble with a shipment of lab reagents. The product arrived with a purity claim that looked fine, but the fine print revealed traces of an unexpected stabilizer. That extra chemical, although not poisonous, threw off his experiment and forced a weeklong delay. This kind of issue can derail research, burn budgets, and erode trust.
Questions to Ask—and Why
When buying anything for scientific use, or even bulk food ingredients, a handful of questions guide the smart shopper. What tests stand behind the numbers? Who carried them out, and under what conditions? Can the creator of the product explain which impurities they saw and which ones they filtered out? Consistency over time—batch after batch—matters more than a single line on a report.
Demanding traceability pulls back the curtain on supply chains. Current best practices call for third-party labs to double-check results. Certificates not only list the numbers, but they also name the method. Is it high-performance liquid chromatography? Gas chromatography? Atomic absorption? Techniques differ in sensitivity and accuracy, so knowing the exact method means you’re not left guessing.
Better Solutions Ahead
The industry keeps moving forward with stronger digital recordkeeping and internet-based verification. QR codes and blockchain tracking let buyers check batch records before even opening a shipment. These tools, once fancy extras, have become must-have features for buyers who want proof, not just promises.
Education ties everything together. As more professionals learn to interpret a specification sheet—linking what’s written to what’s proven—the whole sector benefits. Whether for small high-tech startups or big manufacturers, raising the bar only helps. Each step toward clarity and transparency makes accidental hazards a little rarer.
So, when the question circles back—“What is the purity and specification of this product?”—it’s about a lot more than just numbers. It’s a safeguard, a way to demand honesty and preserve trust. The more people ask—and the better the answers—the safer and smarter the future becomes.
Why Safe Storage Matters
People who work with chemicals know that even a simple slip in storage decisions can mess up months of work—or threaten safety. 1-Ethyl-3-methylimidazolium trifluoroacetate isn’t a familiar name to most, but in the lab, it shows up as a valued ionic liquid for dissolving cellulose, catalyzing reactions, and separating materials cleanly. I spent years as a chemistry student, watching what happens when scientists take shortcuts. A messy shelf isn’t just bad form; mishandling a sensitive reagent throws off experiments and costs real money.
Understanding the Risks
This ionic liquid carries a good deal of chemical stability, but that doesn’t mean you can shrug off proper storage. Over my years training under seasoned lab technicians, I picked up one rule above all: water is always lurking, ready to jump into places you wish it wouldn’t. 1-Ethyl-3-methylimidazolium trifluoroacetate is hygroscopic, drawing in water vapor from the air the moment you leave the cap loose. I’ve seen crystal-clear liquids turn cloudy, and the culprit was almost always moisture sneaking in. Forgetting this for even an afternoon left us with samples that no longer followed expected reaction pathways.
Keeping It Dry and Sealed
Storing this chemical takes some commitment. It thrives in tightly sealed glass bottles. Some labs turn to amber glass to keep stray light from creeping in—not because it breaks down easily under sunlight, but as a habit for prolonging safe use. Parafilm doesn’t cut it; use screw caps with good liners. Everyone on my lab team knew not to trust plastic snap-caps for anything sensitive. Every once in a while, a new researcher would ignore this advice. Within weeks, the once reliable ionic liquid picked up enough water to ruin its performance.
A desiccator, preferably charged with fresh silica gel or activated alumina, becomes its best friend. Tossing it in a regular chemical cabinet exposes it to ambient humidity swings, something even fancy climate-controlled labs struggle to prevent completely. It tells a lot about a lab’s culture—those willing to spend extra care on hygroscopic reagents often keep their data repeatable and their costs down.
Safe Temperatures and Extra Cautions
Room temperature works in most scenarios. Freezers and refrigerators stay out of the picture unless the safety data sheet calls for it. Chemical supply catalogs often list storage at 20-25°C as ideal—enough to avoid unnecessary decomposition and slow any possible reactions with contaminants. Some folks get creative and store all ionic liquids in cold chambers, but frost and sudden temperature shifts add new problems like condensation inside the cap. I’ve mopped up more than a few sticky, ruined bottles after someone tried this shortcut.
Label Everything and Keep Track
No chemical stores itself. Labels with name, date opened, supplier, and any hazard symbols make a world of difference during an emergency—or just a routine audit. It’s easy to forget which bottle’s been open for months if no one bothers to mark it. Tracking batch numbers proved valuable more than once when a supplier issued a recall or when we needed to trace purity issues back to specific shipments. Good habits keep mistakes small.
The Lesson: Proper Storage Pays Off
1-Ethyl-3-methylimidazolium trifluoroacetate doesn’t ask for much—keep it dry, keep it tightly closed, keep it out of direct sunlight, and don’t guess about what’s inside or when it arrived. Each step saves money, prevents risk, and keeps research moving forward. Time after time, I’ve seen that a bit of discipline in storage keeps valuable tools at peak performance, protecting both workers and ideas.
Looking At Hazards and Safety In the Lab
1-Ethyl-3-methylimidazolium trifluoroacetate gets a lot of interest in labs exploring green chemistry and energy storage. I’ve handled this ionic liquid in the past and quickly realized just because it strays from the old chlorinated solvents, it doesn’t hand out a free pass on safety.
Ionic liquids sometimes get a reputation for being “greener” but working with 1-ethyl-3-methylimidazolium trifluoroacetate still means respecting its chemical quirks. Researchers and industrial chemists have noticed its good stability, low volatility, and solid ability to dissolve many kinds of compounds. But none of that means it can be handled like water.
Concerns With Exposure
You don’t want this stuff on your skin. Gloves go on right at the start. Ionic liquids like this one often soak into nitrile and latex, so thicker gloves made of butyl or Viton have treated me best. Long sleeves or lab coats keep droplets off arms, and splash goggles protect eyes from accidental squirts. No one forgets the sting if any drops sneak past safety glasses.
I learned a lot reading safety data sheets and talking to colleagues who test compounds for a living. They say symptoms like skin irritation, eye redness, and coughing show up fast if you get exposed. Having worked with chemicals similar to 1-ethyl-3-methylimidazolium trifluoroacetate, I saw how volatile organic compounds stay in the air in closed lab spaces, causing headaches and even dizziness when air flow isn’t strong enough. Talking with occupational safety officers, they point out this ionic liquid’s low volatility reduces, but doesn’t erase, the risk from breathing in small amounts over time.
Environmental and Health Risks
Working in environmental labs, I’ve seen research suggesting some ionic liquids break down slowly in water, which means they stick around in the soil or wastewater. Long-term, even tiny leaks from rinse water or broken bottles can spell issues for aquatic life because trifluoroacetate, the anion, resists breakdown. I keep spill kits on hand—bentonite clay works well for soaking up small puddles, and I always dispose of waste in sealed, labeled containers. It takes a lot more work than just rinsing everything down the drain.
Oral or inhalation exposure can hit the central nervous system, cause nausea, and disrupt normal cell activity, especially at high doses. That fact alone convinced me to always work with it in a fume hood with the sash pulled low. I keep material on trays, limit batch sizes, and mark everything with clear hazard labels. Basic stuff, but too many people cut corners.
Solutions and Smarter Practices
Good safety starts with honest conversations in the lab. Most accidents I’ve seen happen because someone rushed or treated the new compound too lightly. I like to double-check that all team members understand what to do in case of spills or splashes, and nobody goes solo with unknown chemicals. I also push for testing gloves and lab gear against the compound before we start a project—many surprises come from overlooked incompatibilities.
Technology can help, but habits matter more. Automated pumps limit hand transfers, reducing the chances of direct skin contact. I rely on tightly-sealed bottles with minimal headspace, since less air means fewer vapors. Training never ends—each time a new researcher joins, we run drills, so if something spills or splashes, muscle memory takes over.
Supporting Safe Chemistry
At the end of the day, any progress in chemistry depends on respecting both the promise and risk tied to a compound. 1-Ethyl-3-methylimidazolium trifluoroacetate opens doors in synthesis, battery tech, and extraction, but treating it like sugar can cause real trouble. Keeping an eye on safety data, proper storage, and clear protocols makes sure innovation doesn’t come at the cost of health—or the environment.