1-Hexadecyl-3-Methylimidazolium Chloride: Understanding an Influential Ionic Liquid
Historical Development
Chemistry students and research scientists often point back to the 1970s, that period when ionic liquids started drawing serious attention. Before that, people giving thought to high-melting salts rarely imagined them as alternatives to traditional solvents. The family of imidazolium-based ionic liquids, including 1-Hexadecyl-3-Methylimidazolium Chloride (C16mimCl), entered the scene later. Long-chain alkyl substitutes, like the hexadecyl group in this compound, gave these molecules surfactant-like properties along with their expected ionic behavior. Expansion of green chemistry and the search for low-volatility solvents during the late 1990s and 2000s spurred large-scale synthesis, characterization, and modification work. These years brought new methods that made production less cumbersome and helped make this compound a practical tool, not just a curiosity in the literature.
Product Overview
C16mimCl falls into the category of ionic liquids—materials with distinct salt-like composition that remain liquid near room temperature. This one has a methylimidazolium ring with a 16-carbon chain and a chloride ion. Researchers and industries exploring new surfactants, solvents, and phase transfer catalysts almost always include C16mimCl on their short list. Used frequently in labs, it’s now available from specialty suppliers and found in a growing collection of techniques and applications. The distinctive amphiphilic structure sets this molecule apart compared to simple organic salts or surfactants and has made it a household name among chemists studying self-assembly and colloidal phenomena.
Physical & Chemical Properties
This substance shows up as a white to off-white powder or a viscous, waxy solid, thanks to its extra-long alkyl chain. It won’t dissolve easily in nonpolar organic solvents, but water and many polar organic solvents will handle it. The melting point stands higher than short-chain analogues, usually above room temperature. Once dissolved, the surfactant action springs to life. The cation’s hydrophobic chain helps build micelles, and the chloride anion brings solubility in water. The combination allows it to act as both a detergent and a stabilizer for nanoparticles and emulsions. Laboratory tests reveal remarkable thermal stability and resistance to oxidative breakdown, though prolonged heating or strong bases chew through it eventually.
Technical Specifications & Labeling
Vendors package C16mimCl with purity usually above 97%, listing the chemical formula C22H45ClN2 and a molecular weight near 373.1 g/mol. Shipping labels display hazard information following GHS, including warnings about possible skin and eye irritation, and the caution to handle it in ventilated settings. Certificates usually include analytical results from NMR and elemental analysis, confirming structure and purity. Product data sheets present spectral fingerprints—FTIR, NMR, and sometimes mass spectra—so researchers can spot impurities or isomeric contamination. Shipping involves tightly sealed containers to block out moisture, as exposure leads to clumping and handling difficulties.
Preparation Method
Synthesis starts with alkylation of 1-methylimidazole using 1-bromohexadecane or 1-chlorohexadecane. In my days of working at the bench, I’d use a polar aprotic solvent like acetonitrile, measure stoichiometric amounts, and stir the mixture for several hours under nitrogen to prevent hydrolysis and oxidation. Successful completion brings a solid or viscous liquid, which is washed and recrystallized with ethyl acetate or acetone. Final drying in vacuum brings out that signature waxy powder. This method, well known in both academic literature and patents, gives reliable yields and scales up efficiently for industrial applications. The byproducts, mostly inorganic salts, get filtered out, and careful purification ensures the product’s cation and anion remain free of color and odor.
Chemical Reactions & Modifications
C16mimCl will tolerate a range of chemical manipulations. Substituting the chloride with other anions—using ion exchange resins—brings new properties. Quaternization reactions change the imidazolium ring, letting researchers tailor the molecule for specific purposes. In the lab, I’ve seen functionalization on the alkyl chain, using various linkers and active groups to connect to polymers, nanoparticles, or surfaces. Thermal stability and mild reactivity of the molecule allow most standard reactions under mild conditions. It handles acid, mild base, and various oxidants without breaking down, but treatment with strong nucleophiles or extended exposure to high pH will degrade the ring or strip off the long chain. These features highlight flexibility for custom applications but demand care when developing new formulations.
Synonyms & Product Names
People familiar with commercial chemical catalogs know this compound under several names: 1-hexadecyl-3-methylimidazolium chloride, C16mimCl, or HMIM-Cl. In research literature, the shorthand [C16mim]Cl appears often. Some suppliers call it Cetyldimethylimidazolium chloride, revealing its amphiphilic personality. Whether purchasing from Sigma, TCI, Alfa Aesar, or another major provider, the variations all point to the same structure: an imidazolium ring bonded to a sixteen-carbon tail with chloride counterion.
Safety & Operational Standards
Years of laboratory work teach that handling substances with charged organic components calls for good ventilation, gloves, and eye protection. Safety data sheets stress skin and eye irritation risks, so splash goggles and barrier protection protect from accidental contact. Inhalation isn’t a big threat unless working with powders in dusty conditions, but open containers draw moisture and can turn solids into sticky, hard-to-weigh masses. Spills wipe up with water, as the salt dissolves readily, but any large quantities belong in chemical waste streams, not down the drain. Proper labeling, segregation from incompatible chemicals, and secondary containment prevent missteps—practices familiar to any well-run lab. Emergencies, rare but real, require eye-wash stations and quick access to emergency showers, especially for workers less experienced with surfactant-like chemicals.
Application Area
The application list grows every year. In my own projects, I’ve used C16mimCl to disperse carbon nanotubes and stabilize magnetic nanoparticles. Emulsion polymerization relies on its surfactant action, building stable latexes for paints, adhesives, and biomedical carriers. Environmental labs prize its extraction abilities, especially for heavy metals and hydrophobic organics in water samples. Catalysis groups turn to it when they need unusual reaction media or built-in stabilizers for nanoparticles. The scope has moved beyond academia, reaching into advanced battery electrolytes, drug delivery systems, and soil remediation. Its place among designer surfactants keeps growing, as industries chase after milder processing conditions and new multi-phase systems.
Research & Development
Academic and industrial labs have invested serious time probing the interface science and self-assembly traits of C16mimCl. Scattering experiments, molecular modeling, and even neutron diffraction help unpack how these molecules order at interfaces and build micelles or bilayers in solution. This research fuels improvements in materials science, letting people make new composites, membranes, and sensors. Pharmaceutical projects explore modified analogues as drug solubilizers, sometimes testing how new counterions or chain lengths change compatibility and absorption. The molecule’s keen ability to form ordered structures in water pushes nanomaterials science as well, giving researchers a handle on controlling size and shape during the synthesis of complex particles or molecular devices. Collaboration across fields keeps the innovation engine running, linking chemical synthesis, process engineering, and technology transfer.
Toxicity Research
Every time a new ionic liquid hits wide use, people want solid data on safety and toxicity. Published studies on C16mimCl highlight the need for caution. In aquatic tests, it shows moderate toxicity to some fish and crustaceans, mainly because the long alkyl chain behaves a lot like traditional surfactants in interfering with cell membranes. Cell culture work suggests it disrupts lipid bilayers at high enough concentrations, though short exposures and low doses often carry low risk. Chronic exposure hasn’t been explored in depth, though many labs keep usage low and focus on disposal and environmental protection. Toxicity screening, both in vitro and in vivo, continues as manufacturers and regulators raise the bar for new chemicals reaching the market. Bioaccumulation and persistence draw close attention, since similar compounds end up concentrating in soils and water treatment plants if not managed.
Future Prospects
People following trends in solvents, nanotechnology, and green chemistry point to C16mimCl and peers as rising stars. Custom-tailoring, either via new counterions or ring modifications, grows the product set yearly. There’s a push to reduce toxicity, leveraging bio-based starting materials or degradable side chains. Broader adoption in energy storage—think batteries, supercapacitors—depends on improving conductivity while holding onto chemical stability. Environmental remediation projects hope to tap its ability to trap heavy metals and break down persistent pollutants. Farther afield, the surfactant world sees new blends and hybrid materials drawing inspiration from the molecular structure, with possible breakthroughs in both performance and sustainability. Commercial success ties to how quickly labs and firms can demonstrate both effectiveness and safety, and demand for new data keeps laboratories and regulatory teams busy.
Getting To Know the Chemical
1-Hexadecyl-3-methylimidazolium chloride, often spotted in chemical research labs, brings a bit of personality to the world of ionic liquids. Unlike most household cleaning products, you won’t find this in kitchen cabinets or at the hardware store. It works quietly in the background, playing a direct role in science and industrial production, the kind of workhorse nature rarely gets headlines for.
Why This Chemical Gets Respect
People working in chemistry keep an eye out for molecules that can change the playing field. This compound shines because it belongs to a group called ionic liquids—salts that don’t solidify at room temperature. Scientists use it as a solvent, meaning it helps dissolve things that would normally be tough to mix. With its strong electrical charge, 1-hexadecyl-3-methylimidazolium chloride jumps into reactions that need both stability and a nudge in the right direction.
Lab work isn’t glamorous, but it’s where this product finds its main job. I remember seeing a batch of researchers churn through countless experiments trying to separate difficult chemicals. Using this ionic liquid, they could break stubborn mixtures. It does a better job than water or oil because it handles polar and nonpolar chemicals, something not many solvents pull off. This isn’t about theory; it’s about getting to results faster and cleaner.
Clean Tech and Catalysis
If the planet’s health matters, the right chemistry decisions do, too. 1-Hexadecyl-3-methylimidazolium chloride steps up in green chemistry—ways of making things that are kinder to the environment. Factories want to stop using volatile chemicals that pollute the air or wash away into streams. By using stable, unreactive ionic liquids, less hazardous waste ends up outside. Less waste means fewer health risks and better air. Researchers found that this compound helps recycle metals and supports more efficient chemical reactions, so raw materials go further. That’s not just science—it cuts company costs and lets industries limit their environmental footprint.
Restless Research and Real Limitations
It’s easy to get carried away with new science, but caution keeps people honest. Handling this chemical, I’ve seen how slip-ups can cause skin and eye irritation. Nobody wants toxic exposure, so gloves and goggles are non-negotiable in the lab. There’s growing pressure to track what happens after disposal, since some imidazolium compounds can linger in the environment. The trick always lies in linking scientific progress with health and safety. Big wins come from smart protocols and strong disposal plans, not shortcuts.
Pushing Innovation
Engineers keep looking for ways to push 1-hexadecyl-3-methylimidazolium chloride into new territory. In energy storage, it helps shape stable electrolytes for advanced batteries. Researchers also use it in drug delivery and even in some water treatment projects. They grab onto its ability to interact with a wide array of substances, setting up new ways to trap bad stuff or deliver good stuff right where it’s needed.
This story isn’t just about a single chemical. It’s about how small changes in the lab trigger progress in industries far outside the research building. Getting people to care about the little details—the compounds that do heavy lifting without fanfare—brings us all closer to faster breakthroughs and safer solutions.
Looking Under the Hood
Sitting in a college lab during a warm spring day, a friend passed around a vial of an oddly thick, almost oily liquid. This wasn’t your usual salt or clear solution—it was an ionic liquid, and specifically, the label read '1-Hexadecyl-3-Methylimidazolium Chloride.' In chemistry, having a grasp on the detailed structure of what’s inside the bottle isn’t just for test questions—it's the key to knowing what that liquid can do, where it might end up, and what it brings to scientific discovery and industry.
Breaking Down the Structure
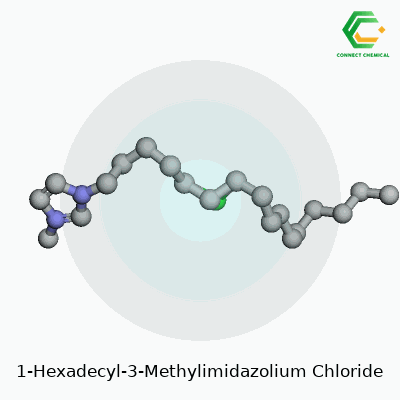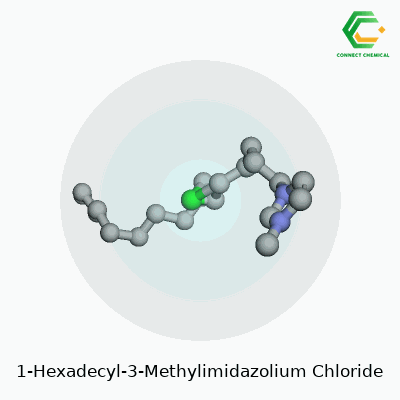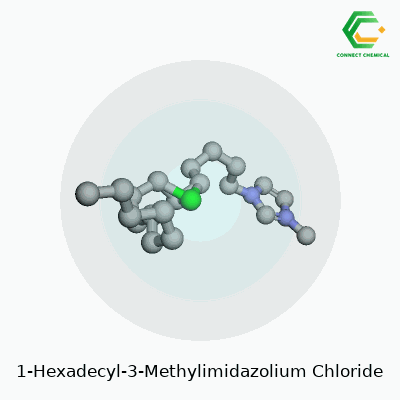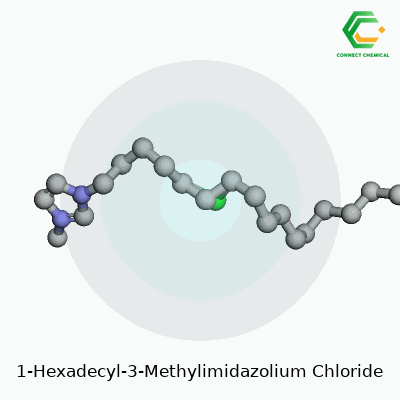
The core of 1-Hexadecyl-3-Methylimidazolium Chloride comes from two parts: the cation and the anion. The cation features an imidazole ring, a five-membered ring containing two nitrogen atoms. One nitrogen in the ring attaches to a methyl group (a carbon with three hydrogens). The other nitrogen forms a bond with a long, straight tail: a hexadecyl group, which is just a string of sixteen carbons ending in hydrogens. This long carbon chain makes the molecule bulky, almost surfactant-like, and highly oil-friendly.
The anion side is the familiar chloride ion: a simple, negatively-charged chlorine atom. The structure forms a salt, but not one you’d sprinkle on fries. The combination packs a punch—an ionic liquid with uncanny abilities to dissolve stuff that usually fights to stay separate.
Why Should We Care About the Structure?
That sixteen-carbon chain does a lot more than just make the name longer. When a molecule wears a chain this long, it doesn’t mix easily with water. This trait matters if you’re cleaning up greasy pollution or separating oil from water. The imidazolium ring brings stability, and sticking on that methyl group dials up resilience, helping the molecule survive tough conditions—heat, acids, even enzymes can’t easily break it down.
In the lab, I watched a beaker turn from cloudy to clear in seconds as this ionic liquid got to work, dissolving dyes and separating oil components. Even in the palm of your hand, the tangibility of these properties hits home. Factories use compounds like this as specialty solvents—turning waste plastics into reusable chemicals, pulling out metals from e-waste, breaking down tough plant fibers for biofuels. As awareness about sustainable production grows, molecules like 1-Hexadecyl-3-Methylimidazolium Chloride draw attention for their role in green chemistry.
Safety, Health, and Responsible Use
Handling unfamiliar chemicals can feel daunting. Ionic liquids often carry a reputation for being safer–less flammable and more stable than old-school organic solvents. Still, the same flexibility making this molecule a chemist’s tool can also raise new questions. While chloride is common, the bulky organic sections don't always break down in nature. Microbes might struggle to chew through the sixteen-carbon chain. Extended use raises possible long-term effects in soil and water. Lab protocols and environmental checks need to keep up. Data from studies over the past decade echo this: some ionic liquids show low toxicity, while others build up in living systems. It takes a critical eye to spot where the risks could tip the scale.
Room for Improvements
Looking ahead, chemists are already tweaking these structures. Swapping the chloride for less mobile anions or adding functional groups that snap apart faster in the environment could make for friendlier molecules. Industry doesn’t just chase performance anymore—regulations and public worry push toward designs that don’t linger or harm life at microscopic levels. Joining hands with biologists and environmentalists, the next batch of ionic liquids will keep surprising us, making everyday processes safer and cleaner.
Understanding What You’re Working With
Lab work throws plenty of chemicals your way. 1-Hexadecyl-3-Methylimidazolium Chloride stands out as one that doesn’t get much attention in the mainstream world, but if you’re dealing with ionic liquids or specialized synthesis, you’ll find it on your bench before long. A long alkyl chain and imidazolium head mix to form a salt used in a surprising range of applications, from catalysis to separation technology. Seeing “chloride” in a name can prompt some extra care, and with good reason. Whenever you see alkyl imidazolium compounds, it helps to remember that safety data sheets should never gather dust.
Risks of Exposure—What Really Matters
The data tells the story here: studies have linked similar compounds to irritation for skin and eyes, and folks who breathe in dust can experience coughing or discomfort. The cation's structure, with its long hydrocarbon tail, raises concern about accumulation in the environment—something green chemistry folks like to talk about when planning research. You’ll see toxic effects on aquatic life in published hazard statements. There’s not a mountain of information on long-term exposure in humans, and you won’t find major regulatory authorities listing it as a notorious toxin. Still, the story isn’t finished; researchers still explore the subtle risks of chronic, low-level exposure.
Face-to-Face with Practical Precautions
When handling this compound, my own approach matches what I learned with most laboratory chemicals: respect beats bravado. I always reach for gloves—nitrile works well for protection. Goggles become non-negotiable, especially if I expect splashing or dust. You want to keep the workspace well-ventilated, preferably with a fume hood. It’s tempting to skip the lab coat when moving between benches, though keeping it on blocks those unplanned moments where splash or spill becomes reality. Make a habit of never pipetting by mouth, even if the quantity looks harmless. Paper towels and water don’t cut it for cleanup; I keep activated charcoal or specific spill absorbents nearby because surface stains can haunt you long after the experiment ends.
What the Science Community Recommends
Safety guidelines written for the bench reflect hard-earned wisdom. Institutional safety programs and chemical suppliers both agree: treat all imidazolium salts as irritants until more data comes out. That means storing the bottle in a cool, dry cabinet, away from acids and oxidizers. Labeling everything precisely saves headaches later, especially if you share space with others. Dispose of waste with guidance from the local chemical hygiene officer, not down the drain. These compounds linger in water systems, and the last thing anyone wants is a note from environmental health about a downstream contamination event.
Building Smarter Habits—Solutions Worth Practicing
Anyone earning their stripes in the lab understands there’s no replacement for staying knowledgeable. Safety training works best when it involves real-life scenarios. During group meetings, I make a point to ask colleagues about recent spills or unexpected reactions. Sharing lessons learned not only avoids repeating mistakes—it reminds everyone that procedural gaps can show up when you least expect them. Keeping digital or paper copies of up-to-date safety data sheets within reach helps less-experienced team members make informed decisions. Regular reminders through posters or short talks push safety culture in the right direction. Consulting reputable sources like the National Institute for Occupational Safety and Health or European Chemicals Agency reinforces the value of evidence-based choices.
Final Thought—Respect the Unfamiliar
Working with 1-Hexadecyl-3-Methylimidazolium Chloride means treating unknowns seriously. Knowledge builds confidence, but a healthy sense of caution keeps everyone working another day. Protective gear and shared expertise create an environment where risks get managed, not just tolerated.
Introduction to Safe Chemical Storage
Working in a lab or handling specialty chemicals in industry means more than just keeping things tidy—it means knowing how even small details can affect results and safety. 1-Hexadecyl-3-Methylimidazolium Chloride, often used as an ionic liquid or surfactant, needs careful attention to storage, both for quality and for health. Plenty of people, myself included, have learned this the hard way by running into clogged bottles, odd smells, and, in some cases, a splitting headache after a poorly stored chemical went bad.
The Role of Temperature
Room temperature does not always mean safe temperature. This compound handles room temperature well, but as soon as it gets too warm above 25°C, especially in humid spaces, clumping or unwanted reactions slip in. Fluctuating temperatures mess with purity. During one summer in a small research facility, uncontrolled air conditioning led to condensation inside sample vials. The contents degraded faster than the experiment. Keeping it in a climate-controlled cupboard or cold room, away from direct sunlight, makes a difference in the compound’s lifespan.
Moisture Control Matters
High humidity speeds up problems for chloride salts. 1-Hexadecyl-3-Methylimidazolium Chloride is hygroscopic, which means it pulls water from the air. Water changes its appearance—usually clumping or even partial liquefaction—wrecks its performance, and sometimes encourages microbial growth. My own short-cut with a loosely capped container led to a bottle full of trouble in under a week. An airtight, well-sealed glass bottle with a screw cap works best. Some labs go a step further by using a desiccator cabinet. Including a few packets of silica gel or another drying agent in storage keeps the humidity down.
Away from Light and Reactive Substances
Most specialty salts do better in opaque containers or dark storage. Light can speed up changes at the molecular level. Chemicals, like acids and oxidizers stored on the same shelf, pose new risks. Accidental spills or vapors mixing nearby push things toward disaster. Dedicated shelves separated from oxidizing agents and acids lower the odds of contamination. At home, I set up a shelf just for ionic liquids and made sure every container got its own label and date. Tracking shelf life kept surprises to a minimum and my records clear for audits.
Personal Safety
Direct contact with skin turns any careless moment into an accident. This chloride-based ionic liquid can cause irritation to skin and eyes. Always wearing gloves, goggles, and even a simple laboratory coat acts as a safeguard. In workspaces with high volumes, annual chemical inventory checks catch outdated or compromised stock before it’s used. This habit once saved a colleague from using a degraded sample that could have spoiled a month’s worth of results.
Disposal and Emergency Planning
Any spill—even a small one—should be cleaned up using appropriate spill kits meant for ionic liquids and chloride salts. Using water to wash an accidental spill may worsen the spread or activate unwanted side reactions. Well-marked Material Safety Data Sheets (MSDS) should be accessible, with emergency eyewash stations and showers nearby.
Final Thoughts on Best Practice
Getting chemical storage right means keeping things simple and consistent. Sticking to manufactured guidelines, separating reactive substances, locking humidity out, and logging inventory set a clear path for success in lab safety and experimental reliability. The right storage solutions protect not only materials but also researchers, saving everyone from unnecessary headaches—sometimes quite literally.
Understanding the Chemical
1-Hexadecyl-3-Methylimidazolium Chloride sounds like a mouthful to non-chemists. In labs, people use it as an ionic liquid, often exploring its unique ability to help separate and dissolve substances. Research in environmental chemistry, catalysis, and even pharmaceuticals sometimes relies on chemicals like this to run experiments or develop new techniques.
For someone shopping for this compound, it’s helpful to know that trade restrictions and regulatory controls keep tight tabs on chemicals like this. Not every country treats sales and handling of it the same. In my own research days, the first step always included checking if a supplier had proper certifications and followed guidelines for storage and transportation. Getting it wrong risks more than a failed experiment; it invites investigations, penalties, or worse.
Where to Start Your Search
Specialty chemical suppliers tend to dominate the market for this material. Well-known companies such as Sigma-Aldrich, Fisher Scientific, and Alfa Aesar list it in their catalogs. You won’t find 1-Hexadecyl-3-Methylimidazolium Chloride sitting on a shelf at the local hardware store, and for good reason. Only qualified buyers with legit commercial or academic business can actually place an order.
Each supplier demands proper documentation. Reputable chemical sellers ask for end-use declarations, proof of affiliation, and regulatory compliance. During my time as a graduate student, our procurement office handled these purchases, sending paperwork back and forth, ensuring every i got dotted. Even now, colleagues tell me about extra checks surrounding chemicals with dual-use status.
Why Strict Controls Matter
Anyone handling a specialty chemical has to respect safety and security rules. Mishandling ionic liquids leads to exposure risks, environmental contamination, or legal trouble. Not so long ago, a university lab made local headlines for lack of safety protocol with a similar imidazolium salt. Cleanup took time, regulators swooped in, and lab staff faced suspensions.
The lesson is clear: strict controls protect not just buyers, but communities and the environment around them. Reputable vendors stay in business because they follow the rules. In my experience, going direct to a recognized supplier keeps paperwork headaches to a minimum and gives a direct line to technical support.
Steps for Prospective Buyers
- Have the right credentials. Suppliers demand proof you belong to a legitimate institution or company.
- Be ready for paperwork. Some vendors even check for environmental health and safety training records.
- Keep up-to-date with local regulations. Some jurisdictions require import or storage permits for ionic liquids like this one.
- Request a certificate of analysis and safety data sheet before committing to an order. Knowing the purity can save a project from setbacks.
Plenty of sellers advertise chemicals online, but my advice is to stick with those who display clear regulatory compliance, robust technical support, and transparent sourcing. There’s little room for shortcuts in chemical procurement, and those who cut corners tend to pay the price down the road. Responsible buyers look out for their teams, their institutions, and their communities.