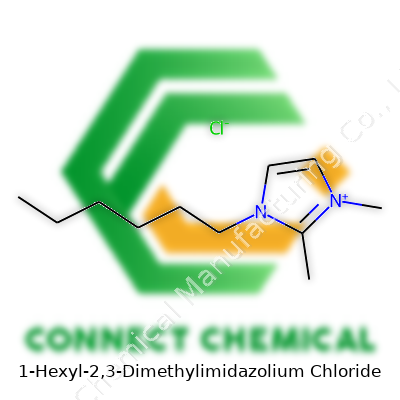
1-Hexyl-2,3-Dimethylimidazolium Chloride: An In-Depth Look
Historical Development
Chemistry has shaped a lot of what we know about modern materials, energy storage, and industrial process design. The arrival of ionic liquids in laboratories opened doors previously closed by flammable or volatile solvents. Among these, 1-Hexyl-2,3-Dimethylimidazolium Chloride started drawing attention during the early 2000s, as researchers searched for safer replacements for conventional organic solvents. The imidazolium family came up in publications, with chemists drawn by their low volatility and heat resistance. This specific compound emerged as a favorite for studies in extraction and catalysis thanks to its unique structure: the imidazole core binds well with alkyl groups, making it easier to break down certain molecules and create custom solvent blends.
Product Overview
Sold as a white or off-white crystalline solid, 1-Hexyl-2,3-Dimethylimidazolium Chloride plays a key role in specialty solvent applications. Researchers and industry both rely on it as a non-flammable, high-thermal-stability liquid. Labs keep it around because it combines ionic character, strong solvating properties, and chemical compatibility with a broad range of organic and inorganic molecules. You might spot it in specialty chemical catalogs under various forms, sometimes supplied as wet or dry powders for ease of handling in custom synthesis.
Physical & Chemical Properties
This molecule boasts a melting point usually above room temperature, letting it function both as a solid and liquid depending on lab conditions. It dissolves well in water and polar organic solvents, but the long alkyl chain means it can also blend easily with less polar chemicals. Its stability under heat and tolerance of strong acids or bases makes it a good choice for harsh chemical environments. The chloride anion offers additional reactivity, supporting both ion exchange and catalytic activities. Labs value this robustness as it enables repeated use across multiple cycles, something traditional solvents can’t always match.
Technical Specifications & Labeling
Bottles arrive labeled with purity, typically above 98%, and detailed handling recommendations. Technical sheets list water content, color, and potential traces of remaining raw materials, as even small impurities can disrupt sensitive reactions. Packaging often involves moisture-tight materials since the chloride ion can take water from air, potentially changing the product’s performance. Shipping standards limit quantities, and suppliers usually highlight safety data sheets detailing storage conditions, hazard classification, and first aid instructions.
Preparation Method
The usual route to 1-Hexyl-2,3-Dimethylimidazolium Chloride begins by alkylating 2,3-dimethylimidazole with 1-chlorohexane, using suitable solvents and controlling temperature to favor the desired substitution. Purification involves washing out unreacted materials and isolating the chloride salt, sometimes by precipitation or solvent removal under vacuum. Chemists check spectra and chromatography profiles to confirm purity before drying the product thoroughly. Lab workers pay close attention to removal of polar by-products, as these can disrupt future work and even corrode equipment over time.
Chemical Reactions & Modifications
This compound acts both as a reaction medium and a reagent. Its ionic structure makes it perfect for supporting various transformations, like metal-catalyzed couplings and acid-catalyzed rearrangements. Changing the anion by metathesis leads to other imidazolium salts with different properties, supporting custom solvent design for new reactions. It supports mild or aggressive modifications based on conditions: sometimes chemists swap the chloride for borate, nitrate, or tetrafluoroborate. Small tweaks to the alkyl chains affect polarity, melting point, and solubility—details chemists exploit when creating new ionic liquids for battery or extraction work.
Synonyms & Product Names
You can find this chemical under several names: 1-Hexyl-2,3-dimethylimidazolium Chloride, [C6C1C1Im]Cl, and sometimes as Hexyl Dimethylimidazolium Chloride. Suppliers across regions list these names interchangeably, so reading chemical formulas matters when sourcing the right material. Chemical reference tools and catalogs help keep track of synonyms during safety checks or cross-supplier ordering.
Safety & Operational Standards
Handling 1-Hexyl-2,3-Dimethylimidazolium Chloride requires care. Users wear gloves, goggles, and store it away from heat and light. The chloride ion makes it corrosive toward some metals, and minor skin or eye irritation happens on contact. Spills get mopped up with absorbent material and disposed as hazardous waste. Safety Data Sheets specify safe storage, spill procedures, and emergency contacts, with many workplace safety departments keeping extra training for anyone handling large quantities. In my experience, regular lab reviews of handling guidelines prevent mishaps and keep processes running smoothly.
Application Area
Researchers reach for this compound in separation science, catalysis, organic synthesis, and increasingly in electrochemical devices. Industrial processes use it in rare earth extraction and as a supporting electrolyte in battery R&D. Some teams develop task-specific ionic liquids, blending variations of these imidazolium salts to optimize selectivity and speed for reactions that previously ran with volatile, toxic solvents. In my own work, swapping conventional solvents for this material lowered solvent emissions and cut down on process changes. It delivers high yield and selectivity in tasks ranging from alkylation and esterification to advanced electrochemistry.
Research & Development
Labs keep tweaking the recipe, looking for new derivatives with custom melting points or solubility. Machine learning shows up now in screening for ionic liquids suited for rare metal extraction and CO2 capture. Publications report improvements in catalysis and separation science, with this chloride salt serving as both a benchmark and a springboard for new designs. I’ve seen academic groups win competitive grants by showing how their ionic liquid blends outperform older processes—resulting in cost savings, reduced hazards, and better process control.
Toxicity Research
Biocompatibility testing for 1-Hexyl-2,3-Dimethylimidazolium Chloride has shown both promise and caution flags. Some aquatic studies point to persistence and moderate toxicity, especially toward small organisms like daphnia. Chronic exposure data remains slim, and regulatory agencies push for new assessments as production increases. Companies have set up in-house programs to evaluate safe limits, environmental impact, and waste treatment. Proper handling, closed-system use, and robust waste treatment reduce risks for both people and the natural world.
Future Prospects
Chemistry rarely stands still. Trends in green chemistry drive innovation with ionic liquids like this one. The quest for safer, more sustainable processes shapes future uses in batteries, biomass refinement, and pollution control. With better data, manufacturers can develop more biodegradable or recyclable forms. Policy-makers, researchers, and industry join forces to balance performance, cost, and safety. Progress relies on real-world testing and sharing best practices—something my field has learned from past mistakes as well as success stories.
Getting Down to What 1-Hexyl-2,3-Dimethylimidazolium Chloride Actually Does
Everyday folks rarely think about ionic liquids like 1-Hexyl-2,3-Dimethylimidazolium Chloride. Yet, researchers, engineers, and commercial labs keep finding new jobs for it. Born out of a push to make solvents that skip the mess and dangers of traditional ones, this compound carries advantages that catch the eye across fields, from chemistry labs to bigger facilities.
Green Chemistry and Cleaner Chemical Processes
Traditional solvents often leave behind waste that can’t just be swept under the rug. By switching to ionic liquids such as this one, chemists trade volatility and flammability for something safer. These liquids stick around under heat, so they don’t vanish into the air or light up with a stray spark. For researchers focused on catalysis — reactions that move along with a little help from a chemical “coach” — 1-Hexyl-2,3-Dimethylimidazolium Chloride brings both stability and a weaker environmental punch.
Lab workers value this ionic liquid for its knack at dissolving a broad range of stuff — from salts to metals — even at room temperature. People working on metal extraction mix it into process fluids when recovering precious metals from scrap. According to a 2022 review in Green Chemistry, researchers used this compound in dissolving and separating palladium and gold, boosting recovery while skipping the harshest acids.
Battery and Electronics Work
Anyone watching electric cars and renewable energy grow can appreciate better batteries. That need means safer electrolytes that hold up through charge after charge. This ionic liquid doesn’t catch fire like typical organic solvents, making it promising for lithium-ion and next-generation batteries. It isn’t just about safety; the liquid’s chemical stability lets batteries run longer before wearing out.
Lab tests show 1-Hexyl-2,3-Dimethylimidazolium Chloride helps carry ions through a battery, improving energy retention. Even in supercapacitors — power sources used for quick bursts of energy — this compound keeps things steady and high-performing.
Supporting Greener Tech in Materials Science
Creating new materials often means working with polymers and nanoparticles. This ionic liquid can act as a swelling agent or dispersant, letting scientists mix and prepare nanomaterials with more control, says a 2023 report from the Journal of Materials Chemistry. In real-world terms, this has helped labs create more uniform coatings and films, key in everything from solar panels to water-repellent treatments for glass.
This compound, due to its low vapor pressure, makes the workplace safer. In workshops where precision thin films go on semiconductors, workers breathe easier and waste shrinks, since fumes barely escape into the air.
Issues, Facts, and Room for Improvement
This compound reduces some classic environmental headaches, but questions about biodegradability and cost remain. Disposal routes for ionic liquids haven’t fully caught up to commercial use. Some, like chemist Robin Rogers at the University of Alabama, point out that scale-up often exposes hidden risks, including long-term effects tied to new chemicals. More eco-focused chemical engineers call for life-cycle testing and tighter regulations so industry doesn’t swap one pollution problem for another.
Better recycling and recovery methods, along with open toxicity studies, would let more people safely use ionic liquids. Efforts to create bio-based versions reflect a growing focus on sustainability.
Why This Matters
Researchers and technicians want options that mix safety, performance, and responsibility. 1-Hexyl-2,3-Dimethylimidazolium Chloride checks important boxes today. The push for honest discussion, ongoing research, and sensible regulations can keep its promise alive as science marches forward.
Why Storage Practices Matter So Much
Over my years working with pharmaceuticals and supplements, I have seen products lose potency just from a sunny shelf or a sweaty transport truck. It’s never just about slapping something in a warehouse and hoping for the best. Even small missteps can lead to spoilage, contamination, or worse—an ineffective product reaching a customer who counts on it. According to the World Health Organization, almost 25% of vaccines globally lose effectiveness because of improper temperature control during storage or transport. That’s not a small risk.
Temperature Makes or Breaks Product Integrity
Every product, whether medicine, food, or a chemical, has its sweet spot. Insulin, for example, demands consistent refrigeration between 2°C and 8°C. If left outside those bounds, even for a short while, it can break down and lose its life-saving properties. Heat and light are silent enemies. I remember working in a retail pharmacy one July—our shipments arrived late, boxes black from baking in the sun. Some children’s antibiotics went back to the supplier, because we couldn’t guarantee their safety. For those of us handling these kinds of goods, temperature logs and backup power for fridges aren’t optional; they’re basic professional responsibility.
Humidity Follows Closely Behind
Excess moisture encourages bacterial and fungal growth. Powders clump, tablets can erode, packaging can warp or become a pathway for contamination. In the food service business, humidity snuck up on us more than once. Silica packets or clever packaging can only do so much. Hygrometers and proper ventilation systems become the difference between safe, shelf-stable goods and wasted inventory. Keeping a storeroom below 60% relative humidity, along with regular inspections for leaks, kept us out of trouble far more than any sticky note reminders.
Cleanliness and Cross-Contamination
In any warehouse, no matter how careful staff think they are, dust and residue build up over time. Chemical and food producers have learned too many hard lessons about allergens and toxins hitching rides on unwashed hands or shared equipment. I’ve seen how the FDA’s focus on current Good Manufacturing Practices (cGMP) means regular, documented cleaning schedules, and clear separation between bulk ingredients. Staff training goes deeper than a few slides—anyone handling sensitive goods needs to know the risks, not just the rules.
Solutions and Technology: Staying Ahead of Risks
Cold-chain logistics offers remote temperature monitoring, giving managers and clients real-time alerts if any product leaves the safe zone. Smart labels that change color when storage conditions slip have prevented close calls where no one noticed the error until it was almost too late. RFID tracking allows better recall management if something goes wrong. None of this is cheap, but the costs of a recall or sick consumer are much higher.
Protocols for the Real World
Product safety relies on more than just good intentions. Industry standards call for temperature logs, calibrated thermometers, lockable storage, and written inventory procedures. Regular staff audits and surprise spot-checks catch problems early. Even in small businesses, basic investments—like reinforced shelves, posted reminders about handwashing, and reliable air conditioning—can have outsized payoffs.
Every Step Counts
Products with specific handling requirements demand respect for science and practical know-how. Each bottle, carton, or vial tells its own story—if handled right, it’ll reach its purpose, whether that’s nutrition, healing, or industrial production. If mishandled, all the technology and quality checks upstream mean little. My own experience, along with countless industry cautionary tales, proves that storage isn’t just an afterthought—it’s where quality assurance finishes its race.
What Are We Dealing With?
1-Hexyl-2,3-dimethylimidazolium chloride isn’t a household name, but it shows up in some corners of research and industrial labs. Folks in chemistry recognize these ionic liquids for their usefulness—a lot of solvents, specialty syntheses, or even as electrolyte solutions in batteries. The appeal often comes from low volatility, which reduces risks from easy evaporation. Tossing around a liquid that doesn’t just float off into the air makes daily lab life less nerve-wracking. Still, invisible dangers live in the details.
Toxicity: Beyond the Surface
No chemical walks into your workspace free of risk, no matter how new or trendy. Early reports on these ionic liquids suggested they might be “green,” but a closer look throws up red flags. Animal studies and cell-based work suggest this compound’s toxicity depends on how much and how often it’s involved. Some research on related imidazolium salts points out adverse effects on aquatic life. For instance, these substances harm freshwater organisms at surprisingly low concentrations, which makes improper disposal pretty problematic.
Personal stories from researchers remind us that skin exposure can irritate pretty quickly, and just because a compound skips the typical “solvent smell” doesn’t mean it won’t cause breathing problems. Ionic liquids like this one can pass through gloves if you don’t double-check their resistance charts. Many underestimate how easily these cations and anions slide through common nitrile gloves. So, even those who keep things tidy end up with headaches or skin rashes after some messy spills.
Real-Life Safety Concerns
Lab manuals might list “low volatility” like a safety blanket, but if someone spills a few milliliters on the benchtop, that stuff isn’t exactly waiting to be mopped up; it’s sticky and tough to clean. What’s worse, its long alkyl chain can make it more hydrophobic and stick around in biological tissue. Research points to possible action on cell membranes—breaking up their barrier function. Researchers worry about chronic exposure. Tiny amounts can build up, affecting enzyme function or causing oxidative stress. Tossing the leftovers down the drain spells trouble for municipal water systems as they aren’t set up for ionic liquid breakdown.
Nobody wants to fuss every time a compound hits the cart, but 1-hexyl-2,3-dimethylimidazolium chloride doesn’t belong with kitchen cleaners. Chronic exposure issues, environmental persistence, and acute effects on living things keep popping up in studies. I can remember a few times cleaning a workspace where this and related chemicals had gotten loose, and days later, folks still complained of faint irritation that just wouldn’t let up.
Addressing the Hazards
Everyone working with ionic liquids needs more than a dust mask and chemical goggles. Double gloving with highly resistant materials, investing in real lab ventilation, and storage away from organics and oxidizers keep things safer. Training should stress how these “mild” looking compounds have hidden teeth. Disposal requires special attention: collect every drop in specific hazardous waste containers, and never tip them into common sinks.
Looking up toxicity data before and after experiments helps inform safer methods. Universities and companies using these substances should invest in better personal protective equipment, standardized handling protocols, and awareness campaigns. There’s no shortcut for safety—especially with substances that cause harm at low concentrations and take ages to break down in the environment. The best labs foster a culture where handling ionic liquids means never letting up on caution or respect for the unseen risks.
Understanding Purity: More Than a Number
I remember standing in a cramped lab as a student, squinting in confusion at a label on a bottle of white powder: “99.7% pure.” At the time, I only cared whether I’d spill any. Years later, I see that tiny number often shapes entire industries — and sometimes health outcomes.
Most people don’t look beyond the label, but asking about chemical purity isn’t nitpicking. It’s the key to knowing whether a substance will actually do the job it’s meant for, whether that’s fueling a car, making medicine, or running a battery.
The Difference a Decimal Makes
The difference between 99% and 99.99% might not seem obvious, but even a fraction of a percent can mean the difference between working reliably or introducing unexpected risks. In pharmacy work, for example, tiny impurities can sometimes trigger allergic reactions or mess with how well a medicine works. The Food & Drug Administration in the U.S. keeps tight restrictions for this reason, requiring careful documentation of every material used in a drug.
Out in the field, chemical engineers in electronics know that microchips rely on some of the cleanest silicon on Earth — purity levels up near 99.9999%. Even a speck of the wrong element can turn a $10,000 wafer into landfill. Precision matters, and so does honesty on the label.
The Problem with Unknowns
Some suppliers cut corners. Testing costs money. Regulations change from place to place. As someone who’s ordered supplies for research, I’ve seen wild variations in claims about what’s inside the bottle. Only a small percentage of buyers ask for certificates or run their own analyses. And sometimes hardly anyone checks except in the case of failure or contamination.
There’s also confusion around what the “purity percentage” actually describes. Are we talking just the absence of other chemicals? Or does that figure ignore water or other solvents left inside? Without clear definitions, buyers risk guessing what they’re really getting.
Paving the Way for Better Practices
A trustworthy product starts with transparency. Using third-party labs to verify contents helps build confidence. Open records and batch results take away some of the guessing and show respect for everyone down the supply chain. When labs make mistakes public and show how they fixed them, it inspires real trust.
Regular audits and surprise checks help catch problems early, before customers face health or financial headaches. Electronic records make tracking much easier. Education also makes a difference: when teams know how to ask the right questions, their companies make safer choices.
Why We Should Care
Chemical purity runs deeper than a marketing claim or a technical spec. It means safer medicine, sturdier electronics, and greener factories. Whether in the pharmacy or at the bench, everyone deserves to know what’s in the bottle.
Why It Matters to Look Beyond the Usual Ionic Liquids
Over the past decade, the study of ionic liquids exploded. Labs around the world started looking at these salts that stay liquid at pretty reasonable temperatures, hoping they’d help clean up chemical processes or supercharge batteries. Most headlines talked about the big names like imidazolium or pyridinium salts with long, familiar side chains. But research keeps turning up new options, and sometimes these options quietly nudge the field forward.
1-Hexyl-2,3-dimethylimidazolium chloride (often written as [C6dmim][Cl]) rarely grabs attention in general conversation, but it deserves a spot at the bench. This molecule combines a hexyl chain with a couple of methyl groups on the imidazolium ring and keeps chloride as the anion. Although crowds often rally around more basic imidazolium compounds, switching the side chains changes the way the molecule handles heat, water, and its ability to dissolve things—from cellulose to metal salts.
Performance in the Lab
One feature that stands out about [C6dmim][Cl] relates to its melting point. Adding extra methyl groups and a chunky hexyl tail can push that melting point down, keeping the ionic liquid pourable at a wider range of temperatures. That really matters if you’re working with sensitive enzymes or trying to use ionic liquids in electrochemical cells, where temperature control saves the day.
Many labs value the improved thermal and chemical stability these tweaks provide. Imidazolium-based liquids like [C6dmim][Cl] handle exposure to light and air without turning yellow or breaking down as quickly as their less substituted cousins. Chloride as an anion gives it the punch needed for tasks like dissolving complex carbohydrates or facilitating organic reactions that need basic halide chemistry.
Challenges and Real-World Solutions
Some would worry about cost and scale. Specialty ionic liquids with unique structures don’t fly off the shelves. Sourcing grams for research remains manageable, but scaling up for pilot plants or real-world applications calls for some creative thinking in synthesis. Labs can use microwave-assisted reactions or alternative solvents to trim down time and waste while bumping up purity. Partnerships with small specialty chemical vendors also keep costs in check.
As with every innovation in the chemical world, toxicity and biodegradability pop up in any discussion. Early studies indicate imidazolium salts with longer side chains could pose more risk to aquatic environments or show bioaccumulation traits. The hexyl and methyl groups in [C6dmim][Cl] push researchers to keep an eye on ecotoxicity, especially if ramping up labs for larger-scale runs. Testing for toxicity in local labs and looking for ways to recover and recycle these liquids after use helps ease those worries. Using closed-loop processes or membrane extraction technology can catch and reuse expensive or hazardous ionic liquids before they escape into the waste stream.
What’s Next for [C6dmim][Cl]
As someone who’s spent time in labs trying to dissolve stubborn biopolymers, I appreciate the flexibility that structural tweaks bring. [C6dmim][Cl] could make some real difference for researchers customizing solvents for each new project. Customizing the cation’s structure lets chemists tweak solubility or stability for their own oddball chemicals and polymers.
At the end of the day, ionic liquid research stays interesting because it keeps evolving. [C6dmim][Cl] offers a fresh side of imidazolium possibilities for those ready to test all the corners of this field. Bold choices with custom cations and careful follow-up in the lab help keep chemistry moving forward, one beaker at a time.