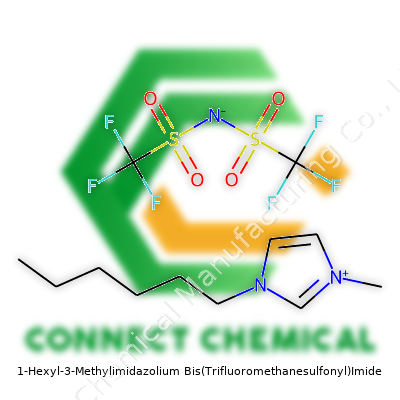
Commentary: 1-Hexyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide—Context, Science, and Purpose
Historical Development
Over the last thirty years, the chemical industry has found itself looking for alternatives to volatile organic solvents. In this quest, ionic liquids emerged as strong options. Among them, 1-Hexyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide (often shortened to [HMIM][NTf2]) grew in popularity. Early research around ionic liquids focused on their unique properties—low volatility, high thermal stability, and tunable solubility—which stood in stark contrast to standard organic solvents that tend to bring safety and environmental headaches. Researchers zeroed in on the imidazolium family after recognizing their broad liquid range. The [NTf2] anion got attention for its hydrophobicity and stability. With patents surfacing through the 1990s and academic labs pushing the fundamental science, industrial players began deploying these salts just as green chemistry began finding its feet.
Product Overview
1-Hexyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide appears as a clear, nearly colorless to pale yellow liquid at room temperature. Users often refer to this chemical as Hexyl-MIM-TFSI or simply HMIM-TFSI, reflecting its cation and anion structure. Its molecular structure consists of a 1-methylimidazolium ring tethered to a hexyl group, balanced by the bis(trifluoromethylsulfonyl)imide anion. Suppliers usually provide this material in glass bottles to prevent absorption of water vapor, which can affect performance. This substance’s unique structure makes it valuable for demanding chemical processes across laboratory and pilot plant settings.
Physical & Chemical Properties
With a melting point usually below –15°C and a boiling point that exceeds 350°C (at reduced pressures), [HMIM][NTf2] offers a genuinely wide liquid range. It exhibits impressive thermal stability, maintaining structure up to 300°C before decomposition kicks in. Its density hovers near 1.34 g/cm³ at 25°C—higher than many organic solvents. This ionic liquid doesn’t evaporate under normal conditions, drastically cutting down exposure risks compared to acetone or toluene. Its moderate viscosity and low vapor pressure also set it apart, allowing tighter control over reactions. Water solubility stays low, thanks to the [NTf2] anion, helping with selective extractions, and supporting electrochemical uses where water content can skew results. The fluorinated anion lends strong chemical resistance against acids and bases, and the imidazolium cation supports hydrogen bond acceptance, which changes how mixtures behave compared to traditional solvents.
Technical Specifications & Labeling
Producers supply [HMIM][NTf2] in high-purity grades—often above 99% purity. Certificates of analysis spell out exact impurity levels, residual halides, and water content, typically all under 100 ppm. Catalogs mention CAS number 155371-19-0 and provide UN numbers reflecting chemical safety. Standard labeling includes batch numbers for traceability, storage recommendations pointing to dry, airtight containers, and handling notices about sensitivity to humidity. Technical datasheets outline properties like refractive index and dielectric constant, equipping researchers with what they need to design experiments or scale-up runs.
Preparation Method
Synthesizing [HMIM][NTf2] often starts with 1-methylimidazole and 1-chlorohexane. When combined under controlled heating, these build the 1-hexyl-3-methylimidazolium chloride intermediate. Chemists then add lithium bis(trifluoromethanesulfonyl)imide, driving a metathesis reaction and swapping out the chloride for the target [NTf2] anion. Post-reaction workup involves repeated washing to strip out byproducts, followed by drying under vacuum. Each batch’s quality depends on tight control over reaction temperature and careful removal of inorganic salts. Researchers constantly seek more efficient and less wasteful ways to make ionic liquids, aiming for lower water contamination and greener methods that skip hazardous halides.
Chemical Reactions & Modifications
[HMIM][NTf2] behaves as an inert medium in many reactions, offering a non-aqueous environment with unique solvation properties. Electrochemists value this liquid for its wide electrochemical window, supporting both cathodic and anodic processes. Catalytic systems can operate in this ionic liquid without catalyst degradation, and it dissolves many metal complexes that struggle in conventional solvents. Modifying either the alkyl chain on the cation or the nature of the anion transforms its properties, letting chemists tailor solubility, viscosity, and cation-anion interactions to fit new reactions. Innovations in anion swapping and cation functionalization open new doors for organic synthesis, extraction, and separation science.
Synonyms & Product Names
Lab literature refers to this compound under different names—1-Hexyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide, [HMIM][NTf2], C6MIM-TFSI, and Hexyl-mim-tfsi all describe the same material. Some catalogs show the older designator 1-hexyl-3-methylimidazolium triflimide or provide abbreviations matched to specific purities or intended uses, like “for electrochemistry.” Buyers should confirm identity with CAS numbers and ask for sample verification, avoiding confusion between similar imidazolium-based liquids with different anions.
Safety & Operational Standards
Working with [HMIM][NTf2] brings its own safety demands. It avoids the flammability headaches of organic solvents, but direct contact causes skin, eye, and respiratory irritation. In the lab, I always wear nitrile gloves and use dedicated glassware to avoid accidental contamination. Users must contain spills quickly, as ionic liquids can damage bench surfaces or sensitive equipment over time. Absorbing significant amounts through skin or inhaling heated vapors can trigger long-term health effects, so good ventilation remains important. Proper disposal follows hazardous waste rules since the fluorinated anion resists environmental breakdown, raising concerns about persistent residues. Safety data sheets point out that although this compound carries lower volatility risk, chronic exposure or improper handling still creates problems, especially in enclosed production spaces. Regulatory frameworks slowly adapt to these newer chemicals, as more data rolls in on their toxicity and environmental fate.
Application Area
[HMIM][NTf2] found early wins in extraction and separation processes, pulling metal ions or organic pollutants from water or complex mixtures. Analytical chemists exploit its non-reactive nature in chromatography and electrochemistry, while process engineers load it into lithium battery electrolytes to boost thermal and cycle stability. Wearable electronics and flexible batteries often draw on its ion-conducting skills. In the lab, I have seen this compound anchor homogeneous catalysis for carbon-carbon coupling and ease biomass conversion, offering an alternative to noxious organic acids. The absence of vapor makes it easier to recover after reactions, saving money and reducing emission worries. It pops up in pharmaceutical research, where controlling crystal forms or enabling gentle extraction can protect fragile compounds. Skyscraper urban farms and clean manufacture support the push to use ionic liquids for safer, lightweight chemical handling—a clear sign of growing industrial confidence.
Research & Development
University and industry teams invest heavily in expanding what [HMIM][NTf2] can do. Ongoing research focuses on greener production, looking for bio-based precursors to cut petrochemical ties. New work explores its behavior with nanomaterials, searching for stable dispersions and functional coatings that demand mild conditions. In my experience, R&D groups love playing with the cation’s side chain to uncover new extraction selectivity or boost battery performance in freezing climates. Measurement teams refine methods for detecting trace impurities, helping industrial partners meet tighter specifications. As demand surges for low carbon processes and higher purity materials, more projects center on closing the loop with effective ionic liquid recycling. Reliable pilot data and transparent communication between academia and industry accelerate these advances.
Toxicity Research
Data on [HMIM][NTf2] toxicity keep trickling in, highlighting both strengths and blind spots. Animal trials show limited acute toxicity, but long-term, chronic exposure paints a murkier picture. Researchers worry about bioaccumulation—especially with fluorinated anions that break down slowly and resist many environmental treatment systems. Waterborne life faces the biggest threat, as even low concentrations can disrupt sensitive aquatic systems. Data from cell studies show possible effects on membrane function, flagging areas for more careful exposure controls. Regulatory groups now push for better lifecycle assessments, asking companies to share end-of-life fate and toxicity management plans. In practice, labs enforce strong containment and limit discharge, building good habits early.
Future Prospects
Changing regulations and customer demand for safer, more sustainable chemicals drive [HMIM][NTf2] toward broader markets. As benchmark solvents hit regulatory walls, labs will look to ionic liquids for cleaner processes. Energy storage, microelectronics, and green processing technologies create huge demand for solvents with tailored features and proven performance. The next wave of innovation will come from tapping renewable feedstocks and closing the recycling loop, limiting escape into soil and water. Chemists who understand the practical risks and design for safety can help lead adoption, keeping the technology out of the “greenwashing” trap by sticking to hard data and clear safety protocols every step of the way.
Digging Into Ionic Liquids
I’ve spent enough time around laboratories and researchers to see the excitement when someone pulls a new bottle from the fridge, and 1-Hexyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide always gets extra attention. Its tongue-twisting name hides its status as an ionic liquid, and these compounds do more than gather dust. Their properties—liquid at room temperature, low volatility, and strong solvating power—offer plenty for chemists and engineers to work with. This one, often called [HMIM][Tf2N], has found itself at the center of everything from greener chemistry projects to next-generation batteries.
Push for Greener Chemistry
Chemists and environmental regulators grow more uneasy with each shipment of flammable organic solvents. Some solvents pollute groundwater, others burn at the worst possible moments. HMIM-Tf2N earned attention by stepping away from volatility almost entirely. Run a reaction in this liquid, and you no longer deal with most of the harmful fumes that come from ether, chloroform, or toluene. I’ve watched lab groups swap in ionic liquids to cut down on pesky regulatory checklists. Sometimes, those changes take a bite out of yield or speed, but often, researchers find a blend of safety and efficiency.
Special Role in Extraction and Separation
Try to pull rare metals out of spent electronics, and it’s easy to get lost in the mess. HMIM-Tf2N enters the scene as a remarkable solvent, helping dissolve stubborn metals or organic molecules that traditional solvents ignore. Because this liquid forms strong bonds with charged species, it picks apart metal mixtures with a surgeon’s precision. Tech companies and recycling startups both experiment with it to pull cobalt, lithium, and rare earth elements out of shredded circuit boards. This doesn’t solve the world’s e-waste problem, but it’s a step forward for recovery rates.
Making Better Batteries
Over several years, battery labs ran into issues with safety and performance as lithium-ion designs advanced. HMIM-Tf2N brings thermal stability and nonflammability, which matters when packing batteries together in tight spaces. Some lithium-ion batteries use this compound as the basis for their electrolytes. It keeps ions moving without catching fire or generating harmful gases. As electric cars and portable electronics push battery demands higher, safer electrolytes keep lives and products intact during accidents or severe weather.
Room for Growth and Challenges
No tool in chemistry comes without questions. Producing HMIM-Tf2N calls for specialized reagents and processes, making it pricey and sometimes tough to scale. Excellent thermal stability helps in the lab but complicates breakdown or disposal outside controlled spaces. If the technology moves out of the lab and into industrial-scale use, handling and recycling will have to catch up. Conversations in conferences focus on lifecycle and cost; nobody wants to trade one environmental headache for another.
Ideas Worth Pushing
Researchers tackle these challenges by closing loops and building recycling methods directly into process planning. Companies experiment with recovering used ionic liquid from process streams, or reformulating mixes to boost recyclability. Academic labs chase plant-based or more degradable alternatives, but chemistry rarely offers perfectly clean answers. As one part of the push for safer, more efficient manufacturing, HMIM-Tf2N doesn’t solve everything, though it certainly earns its spot on the shelf of anyone aiming for greener tech or advanced energy solutions.
The Real-World Impact of Storage Mistakes
Most of us have opened a box of food, paint, or medicine and realized something’s off. Maybe the crackers taste stale or the odor from a bottle doesn’t seem right. Small changes in shelf life might not seem like a big deal, yet for many products, including foods, pharmaceuticals, and even batteries, storage really shapes quality, effectiveness, and sometimes safety.
Temperature: Not Just a Number
I’ve worked in places where labels asking for “cool, dry storage” get shrugged off. Folks might stash items by a window or in a stuffy closet. Products exposed to heat break down faster than most realize. For example, heat can turn chocolate into crumbly white lumps and wreck a bottle of aspirin by speeding up chemical changes. According to the World Health Organization, a lot of medication loses potency fast above 25°C. Food begins to spoil when temperature climbs, even in sealed bags. Regular room temperature – about 20°C to 25°C – fits most dry, shelf-stable goods, but few food pantries stay steady all summer.
Moisture: The Quiet Culprit
Humidity creeps into everything. When stored in damp basements or refrigerators packed with condensation, dry products like cereal or spices soak it up and go soft or moldy. Electronics also suffer. Moisture triggers short circuits and corrosion. According to U.S. Department of Agriculture data, grains held at more than 60% humidity sprout fungus quickly. I once stored flour on a basement shelf—not realizing a tiny leak left the air damp—and had to toss everything after weeks of storage. Desiccant packs and tight seals help, but location always matters most.
Light Exposure: More Than Just Fading Labels
You’ve probably seen vitamin bottles or medication in brown or dark blue glass. Light, especially sunlight or strong store lights, breaks down sensitive compounds. I saw boxes of paracetamol fade from white to yellow after sitting in a sunny stockroom window. UV-sensitive products, including many vitamins, lose their strength much faster under bright lights. Opaque containers keep products fresher, and for home storage, a cupboard or closed box keeps light-sensitive items safe from direct rays.
Air Exposure: Oxidation Damages and Spoils
Ever noticed chips go stale after the bag’s been open a day? Oxygen stirs up chemical reactions. Fats turn rancid, colors fade, and medicines lose their punch. After opening, seal the bag or bottle right away. For longer shelf life, products like coffee beans or certain ointments sit better in containers flushed with an inert gas or vacuum-packed. Large manufacturers often use these tricks for a reason—air speeds up spoilage every time.
Practical Advice for Everyday Storage
Most storage failures stem from rushing or guessing. The product packaging gives the best hints, as required by regulations: “Keep in a cool, dry place,” or “Protect from light.” A pantry far from the stove, a closet away from pipes or outside walls, or a drawer out of the sun keeps most products safe. For sensitive items, always double check the label, ask a pharmacist, or look for the manufacturer's details online. Using common sense—keeping things dry, shaded, and at a steady temperature—stretches shelf life, prevents waste, and protects health.
Understanding Hazards Hiding in Plain Sight
Plenty of folks have felt that uneasy moment standing in the aisle, squinting at an ingredient label, and wondering if a product on the shelf could spell trouble down the road. Safety slips into daily life in simple forms—a cleaning spray at home, a beauty cream, a new gadget fresh out of the box. As someone who learned to read labels after a family member’s allergy scare, I know how fast a product’s risks can go from abstract to urgent.
Smart Questions: More Than a Legal Label
The “hazardous” tag doesn’t only live on chemical drums or power tools. Even a small bottle of hand sanitizer can trigger a trip to the ER for a toddler. The Consumer Product Safety Commission tracks stories like these, logging thousands of hospital visits caused by stuff you’d never suspect—laundry pods, faulty batteries, perfume, certain plastic toys. Product recalls pop up in headlines, showing that the system sometimes drops the ball until it’s too late.
Facts, Not Hype—What Makes Something Dangerous?
Most risks don’t hide behind mystery science. Sometimes, companies pack cleaners with harsh solvents that sting the lungs or leave dishwasher tabs loosely packaged and easy for kids to swallow. Flammable sprays lined up under the kitchen sink or lithium-ion batteries in e-cigs earning headlines for small explosions—these cases prove hazards come in many forms. In 2023, over 200,000 children in the U.S. landed in emergency rooms because of accidental exposure to household products, according to the American Association of Poison Control Centers.
People with allergies, pets, or asthma feel the risk in their pockets and lungs. Scented candles or air fresheners can quietly fill the house with compounds that trigger migraines or breathing trouble. I’ve tossed more than one candle after it set off my daughter’s asthma. Even “natural” products can cause harm, as poison control lines will tell anyone who had an adventure with essential oils left within a toddler’s reach.
Where Companies Trip Up—And How Consumers Can Push Back
Many safety issues grow out of shortcuts and fuzzy rules. Some products hit shelves with minimal testing, especially with online marketplaces shipping goods directly from factories around the world. Oversight agencies don’t always have the muscle to dig through mountains of new gadgets and chemicals arriving every day. This leaves people relying on warning symbols, vague instructions, or word-of-mouth from friends who already learned the hard way.
Transparency changes everything. Labels that actually warn about specific risks—chemical burns, choking, flammability—make a difference. Tracking down batch numbers and finding out if something got recalled seems basic, yet recall notices slip past most people who actually own the affected products.
Real Solutions Take Teamwork
Nobody gets full peace of mind by reading fine print alone. Trust builds when brands explain ingredient lists in plain language and regulators actually enforce rules with surprise checks. Stores can step up too, pulling risky goods off shelves before someone gets hurt. Regular updates about safety trends and real consequences for hiding risks send the message that safety deserves front-row attention.
In my house, careful label reading feels less like paranoia and more like everyday sense. My biggest lesson? Keep asking questions. If the answer sounds vague or dodgy, it’s probably not worth the risk—especially to the ones whose safety relies on us reading between the lines.
Why Purity Levels Shape Industry Choices
Every time I stand in a lab staring at a bottle label, I pay close attention to purity. Even tiny traces of contaminants can ruin results, sometimes in ways not obvious until it’s too late. Chemical purity isn’t an academic concern—it’s the key to predictable results in everything from pharmaceuticals to battery production. When a business wants reliable output, it relies on a well-defined standard of purity. The top grades really matter.
Understanding the Grades
Chemicals show up under a range of grades: technical, laboratory, reagent, and pharmaceutical. Technical grade covers mass-production situations where some impurities just don’t cause problems—think construction, textiles, or automotive tasks. When I see technical grade on a bag of sulfuric acid, I know split-second precision isn’t on the table.
Laboratory and reagent grades raise the bar to satisfy scientific work and quality control. I’ve worked in labs where reagent grade ethanol kept important reactions on track, thanks to its well-documented low contaminant profile. Analytical chemistry, environmental testing, and food inspection all run smoothly only with these cleaner, traceable sources.
Pharmaceutical and food grades hold the line for medications and consumables. Regulations demand more than just purity—they insist on records of every process, every ingredient. The extra cost isn’t just a formality. When patients consume a product, mistakes can ruin lives and reputations. I remember a recall that traced back to low purity in a widely used ingredient; it cost the company millions and sent consumers searching for answers.
How Purity Gets Verified
Standards matter, but the proof is in the testing. Labs use instruments like chromatography and spectroscopy to pinpoint tiny amounts of other elements or molecules in a batch. Regulatory agencies set these limits not only for safety, but also because inconsistent batches slow research, waste time, and rack up costs in product recalls.
Manufacturers publish certificates of analysis, breaking down purity as a direct report instead of just a grade label. This isn’t red tape; it’s essential transparency. Without it, entire sectors—like semiconductors or vaccine development—risk grinding to a halt.
Problems Grow When Standards Slip
I’ve seen companies cut corners, lured by cheaper bulk chemicals that promise “close enough” results. Contamination sneaks in. Machines break. Product batches get scrapped. The cost of rework or regulatory fines often overshadows any up-front savings. In real-world agriculture, even a slightly impure fertilizer might introduce toxic heavy metals into the food chain.
And then there’s global supply. Not every country uses identical standards. Companies importing raw ingredients sometimes gamble on unfamiliar grades or mismatched analytics. Sudden short-term savings become expensive mistakes as production halts, customers complain, or worse—products fail.
Solutions for Safer, Straighter Supply Chains
Accountability helps. Suppliers who open up about their testing, who welcome third-party audits and keep clear documentation, win trust. I have seen this firsthand; the most consistent labs I’ve worked with kept those documents up to date and their processes transparent, never hiding behind vague labels.
Global alignment—standards that few ignore—helps reduce confusion. International organizations have pushed for tighter, consistent policies. Clear communication between manufacturers, suppliers, and end-users closes loopholes that poor-quality products sneak through.
Investment in testing is an upfront cost that dodges disaster in the long run. Strong feedback loops help, too: researchers flagging odd test results, quality managers speaking up, production staff raising concerns. All together, people and paperwork give chemical purity meaning beyond a number printed on a drum.
Understanding the Risks
Dealing with chemicals like 1-Hexyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide doesn’t have to be scary, but it does require respect for what you’re working with. This ionic liquid shows up in labs thanks to its stability and usefulness, especially in solvents and advanced materials research. Folks often don’t realize that just because a liquid is stable, it doesn’t always mean it’s harmless. Inhaling vapors or allowing skin contact introduces risks—trifluoromethanesulfonyl compounds in particular can irritate the lungs, eyes, and skin, even if you can’t see the effects right away.
Personal Protective Equipment and Good Habits
No substitute exists for gloves and goggles here. Nitrile or neoprene gloves do a good job standing up to ionic liquids, while basic latex breaks down too fast. Investing in a reliable splash shield or lab coat doesn’t just tick a box for compliance; it protects against the occasional splash or accidental contact that anybody who’s worked at a benchtop recognizes. Even experienced researchers make mistakes on a long day.
Ventilation often gets overlooked, especially in compact university labs. These ionic liquids can sneak volatile byproducts into your breathing space. If you can’t afford full fume hoods, at least use a well-fitted local exhaust system. You spare yourself a boatload of headaches — literal and bureaucratic — if you think ahead.
Spill Management: Immediate and Careful
Everyone drops something eventually. Absorbent pads and an easy-to-read spill response sheet should stay within grabbing distance. The only smart way to handle a puddle is to surround and soak it up fast with inert materials like sand or commercial absorbents. Never sweep a chemical like this under the rug — no amount of busy work saves you from a floor turned slick or a hidden chemical hazard.
As someone who has had to mop up after a carelessly handled liquid, I recommend double-bagging soaked absorbents and labeling the bag right there. Once the mess ends up in a corner, memories fade and people leave; clear labeling avoids headaches for the next person down the line.
Disposal: Not for the Drain
Regular sinks weren’t built to process this stuff. Pouring it down the drain leads to expensive plumbing problems and local environmental hazards. Many water treatment facilities still struggle to break down novel organic chemicals, meaning they wind up in rivers and lakes a good distance away. In my experience, wastewater authorities take special interest when solvents or exotic ionics turn up somewhere they shouldn’t.
Chemical waste services remain the responsible way. Seal up any leftover liquid or contaminated solids in their own containers, making sure to note the contents precisely on the label. Some places want you to keep halogenated materials separate, so ask your facility manager what’s required. Never guess or trust a hunch — double-checking the disposal protocol saves time and prevents potential fines.
Training and Long-Term Safety
Teaching new lab members about chemicals like this pays dividends. A short demo of proper glove use and spill response sticks in memory much longer than a dry manual. Written protocols make sense but real-world practice roots habits. I recommend a quick session every semester, so muscle memory kicks in when the unexpected happens.
Watching out for long-term health and reducing accidents takes more than one person’s effort. Lab groups that check on each other and update safety data sheets regularly see fewer close calls and less confusion down the line. Everyone has a role in keeping their space safe — and with chemicals like 1-Hexyl-3-Methylimidazolium Bis(Trifluoromethanesulfonyl)Imide, that teamwork pays off in quieter days and cleaner labs.