1-Hexyl-3-Methylimidazolium Bromide: Exploring Origins, Uses, and Future
Historical Development
Ionic liquids, like 1-Hexyl-3-Methylimidazolium Bromide, emerged in the late 20th century, drawing attention as researchers looked for alternatives to volatile organic solvents. Growing environmental concerns drove chemists to rethink solvents for safer, cleaner processes. The efforts around the early 1980s to 1990s started paying off when imidazolium-based ionic liquids came to the scene. Chemists blended practical need with creative synthetic chemistry, shaping a class of materials where molecular structure could be tuned for almost any application. Navigating through decades of chemical journals, one finds that 1-Hexyl-3-Methylimidazolium Bromide showed promise both as a functional ionic liquid and as a scaffold for tweaking other properties. This pursuit became urgent as green chemistry principles gathered force, nudging industry away from flammable, toxic solvents.
Product Overview
1-Hexyl-3-Methylimidazolium Bromide belongs to the family of room-temperature ionic liquids. Its cation, a six-carbon side chain imidazolium, paired with a bromide anion, tailors the melting point and chemical behavior. Chemists value it for excellent thermal stability and low volatility, making it easier and safer to handle than most traditional organic solvents. It appears as a colorless or pale yellow liquid at room temperature, though variations happen due to minor impurities from synthesis. Packaging usually involves tightly sealed amber bottles to shield it from moisture and light, as both can affect quality. Typical purity levels exceed 98%, with moisture levels monitored to prevent unwanted hydrolysis or decomposition in sensitive applications. Common synonyms in research and supply catalogs include HMIMBr, 1-Hexyl-3-methylimidazolium bromide, and its CAS number 171058-18-9. More than just a label, this liquid has found its way into chemical labs, catalysis studies, and even pharmaceutical formulation research.
Physical & Chemical Properties
The hexyl chain imparts certain oil-like properties: 1-Hexyl-3-Methylimidazolium Bromide shows a melting point typically below 40°C and often remains liquid at room temperature. Its density falls between 1.0 and 1.1 g/cm³ at 25°C, heavier than most water-based solutions but lighter than other salts. Solubility stands out—miscible with water, acetonitrile, ethanol, and many polar organic solvents, but much less so with non-polar hydrocarbons. Thermal stability runs high, with decomposition temperatures reaching above 250°C, allowing for reactions that would destroy classical organics. Its ionic conductivity enables electrochemical work. The viscosity lands somewhere between syrupy and fluid, varying with temperature and water content, which influences handling and process design.
Technical Specifications & Labeling
Manufacturers label 1-Hexyl-3-Methylimidazolium Bromide under strict guidelines: key metrics include purity, water content (measured by Karl Fischer titration), and residual halide impurities. Certificates of analysis list heavy metal content, making trace environmental impact or cross-contamination less of a guessing game. For shipping, containers receive GHS-compliant hazard labels noting possible irritation hazards. Material Safety Data Sheets highlight key points like storage below 40°C, tight sealing, and no contact with oxidizers or halides to cut accident risk. Batch numbers, manufacturing date, and recommended shelf life—usually up to two years under dry, dark conditions—appear on every certified bottle.
Preparation Method
Synthesis involves a quaternization reaction: 1-methylimidazole reacts with 1-bromohexane under dry, controlled heat, typically in a polar-aprotic medium such as acetonitrile. The mixture stirs for several hours until the product forms, followed by purification with solvents like ethyl acetate. Washing and drying under vacuum removes residual water and unreacted starting materials, delivering a product that meets high research standards. Yield optimization takes tweaking of time, temperature, and solvent ratios, a challenge faced by many trying to scale production from lab to pilot plant. Some greener syntheses use microwaves or solvent-free protocols aiming to curb environmental cost while maintaining quality.
Chemical Reactions & Modifications
1-Hexyl-3-Methylimidazolium Bromide offers a flexible scaffold for functionalization. The cation can be swapped for other alkyl chains or functional groups, engineering properties for catalysis, extraction, or biomolecule stabilization. Ion exchange swaps bromide with other anions—PF6-, BF4-, ClO4-—expanding phase behavior and reactivity. Environments having water, acid, or base bring hydrolysis or halide displacement, which explains why storage and handling see such emphasis on dryness and chemical compatibility. Researchers have attached catalysts, dyes, or biomolecules through the imidazolium ring nitrogen or the terminal hexyl group, pushing boundaries in chemical separations and material science.
Synonyms & Product Names
Common names pop up across literature and commerce: HMIMBr, 1-Hexyl-3-methylimidazolium bromide, [HMIM][Br], and its abbreviation Hmim-Br. Each identifier tells a slightly different story of its structure and use. CAS 171058-18-9 signals clarity for regulatory or supply chain traceability. Commercial catalogs may also cross-list this product under phrases like “imidazolium ionic liquid (bromide)” to fit diverse user searches. This abundant cross-listing cuts down on confusion for procurement and makes regulatory monitoring smoother.
Safety & Operational Standards
Toxicological studies show 1-Hexyl-3-Methylimidazolium Bromide can act as an irritant, especially to eyes and skin. Lab protocols recommend gloves, goggles, and good ventilation. Its chemical stability means spill risks stay low, but the potential for environmental aquifer contamination sparks debate about lifecycle responsibility. Waste disposal goes through specialty handlers, recognizing the ionic nature and lack of biodegradability in most water treatment systems. Long-term safety research continues in line with REACH and other chemical registration mandates, with annual updates on toxicology data. Emergency procedures drill on eye flushing, dermal rinsing, and isolation of contaminated surfaces, reflecting the push for zero-incident labs.
Application Area
1-Hexyl-3-Methylimidazolium Bromide crosses into fields as diverse as extraction chemistry, electrochemistry, catalysis, and organic synthesis. Solvent applications benefit from its low vapor pressure, cutting down solvent-loss costs and workplace health risks. It supports catalytic reactions, as both solvent and cocatalyst, in hydrogenation, oxidation, and carbon-carbon coupling. In biochemistry, it dissolves otherwise recalcitrant biomolecules, finding a role in protein or nucleic acid extraction from tough cells. Its use in electrochemical cells, from batteries to sensors, comes from its wide electrochemical window and reliable conductivity. Environmental chemists use it to extract heavy metals or pesticides from wastewater for analysis, leveraging its selective solubility and phase-transfer character.
Research & Development
Academic journals brim with studies using 1-Hexyl-3-Methylimidazolium Bromide as a platform for exploring structure-function relationships in ionic liquids. Researchers probe its effect on catalyst activity by varying the alkyl chain, tweaking the electronic environment for maximum effect. Process engineers study scaling synthesis from milliliter to liter scales. Efforts focus on greening the manufacturing pipeline, from reducing solvent loads to recycling spent ionic liquid using custom extraction membranes. Crowded conference halls feature posters on using it for DNA stabilization, enzyme catalysis, and even plastic recycling, pointing to an uptick in cross-disciplinary interest. Industry partners now test it as a sustainable alternative in electronics recycling and advanced materials synthesis, looking for both cost savings and cleaner footprints.
Toxicity Research
Detailed animal and cell studies draw attention to dose, exposure routes, and long-term bioaccumulation. Concerns linger about cationic surfactant-like behavior impacting aquatic organisms, driving tighter guidelines on discharge and worker hygiene. Chronic exposure studies reveal moderate toxicity at high doses—enough to warrant controlled use, but not so high as to block research or synthetic applications. Data gaps on degradation pathways and metabolite behavior push regulatory agencies, like the EPA and ECHA, to demand further studies. Calls grow louder for alternative synthesis and recovery routes, as conventional incineration does not always break down the imidazolium backbone. On the whole, informed use with proper disposal keeps risks in check, but this remains an area in flux as new findings come to light.
Future Prospects
Researchers see ionic liquids revolutionizing chemical industries as solvents, processing aids, and functional additives—if lifecycle challenges can be addressed. Wider adoption of 1-Hexyl-3-Methylimidazolium Bromide and its cousins hinges on cost reduction, scaling green manufacturing, and closing the loop on waste. Hybrid approaches, where ionic liquids work in tandem with enzymes or recyclable solid supports, promise less chemical waste and higher selectivity. Advances in clean-up technology may also unlock broader environmental applications, such as in-site remediation or closed-loop water treatment. As young researchers enter the field, fresh takes on the classic imidazolium structure may yield safer, greener, and more effective options, keeping this class of chemicals front and center in the conversation about sustainable chemistry.
Breaking Down the Structure
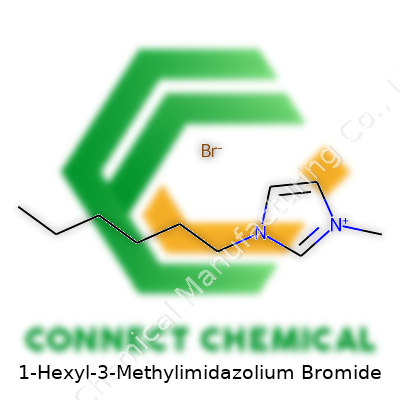
Anyone with a background in chemistry recognizes how important molecular structure is for understanding how a compound behaves. 1-Hexyl-3-Methylimidazolium Bromide, often written as [HMIM]Br, stands as a great example of the new wave in ionic liquids. The molecule combines an imidazole ring with two distinct groups: a methyl on one nitrogen and a hexyl chain on the other.
Let’s picture the core piece: the imidazolium ring. This five-membered ring contains two nitrogen atoms sitting at opposite sides, with three carbon atoms completing the cycle. On the first nitrogen, a hexyl group (six carbons in a straight chain) stretches out, and on the third carbon, a simple methyl group hangs. The entire ring carries a positive charge.
Next to the ring, bromide shows up — a bromine anion, to balance the charge sitting on the ring. That's the basic ionic character of the structure. The ring itself is aromatic, which helps explain the stability and some unique properties of this compound.
Why This Structure Matters
This architecture really matters, especially in the field of green chemistry. The long hydrophobic hexyl chain helps dissolve both polar and nonpolar substances, something not many compounds pull off. That makes 1-Hexyl-3-Methylimidazolium Bromide useful in all kinds of experiments, including catalysis, separation techniques, and materials science.
I once worked on a project looking for alternatives to volatile organic solvents in analytical labs. Traditional solvents put people at risk and escape into the air with real consequences for air quality. Swapping those for 1-Hexyl-3-Methylimidazolium Bromide cut down emissions while boosting extraction efficiency. This wasn’t theory or test-tube science — it changed the working environment for everyone involved.
Issues Around Safety and Environmental Impact
No compound offers a free lunch. While ionic liquids like this one reduce volatility, disposal poses tough questions. The presence of bromide raises red flags for many wastewater treatment groups. Bromide ions can react to form brominated organics, some of which linger in the environment longer than expected. Experts agree that current testing and disposal methods require fresh scrutiny.
Lab safety officers often remind chemists to handle even seemingly benign ionic liquids with care. Skin exposure, inhalation, and improper handling could land someone in the hospital. Data from recent years show that while toxicity looks lower than classic organic solvents, new health effects could show up after long-term exposure. This suggests that researchers need to remain vigilant, update their risk assessments, and push for more complete toxicological studies before rolling out widespread adoption.
Looking for Solutions
The path forward involves research into less hazardous anion-cation pairs. Some chemists already experiment with biodegradable alternatives or non-halogenated ionic liquids to cut down long-term risks. Companies and universities can tweak the side chains or swap out bromide for greener choices, improving overall safety while retaining the beneficial properties.
Collaboration remains key. Industrial chemists, toxicologists, and environmental regulators need open communication to address disposal strategies and design next-generation ionic liquids. None of these changes will happen overnight, but staying informed helps everyone use these powerful chemicals safely and responsibly. In this way, something as simple as a methyl-hexyl ring partnered with bromide points the way toward safer, greener laboratories.
The Value of Ionic Liquids in Research and Industry
Scientists chase new ways to solve old problems. In my own experience working in a lab, experimentation with ionic liquids changed how I looked at chemistry. Among these liquids, 1-Hexyl-3-Methylimidazolium Bromide stands out. This compound blends the imidazolium core with a stiff, hydrophobic tail, balancing properties that prove useful across scientific disciplines.
Pushing Boundaries in Green Chemistry
One major appeal comes from its role as a “green” solvent. Most solvents on lab benches evaporate easily, releasing harmful vapors. This one barely registers on the volatility scale, which means fewer emissions in studies or when scaling up processes for production. That translates to cleaner air in the work environment and lighter regulatory burdens. Research shows that swapping out volatile solvents with this ionic liquid can cut total emissions during chemical syntheses.
Breaking Through in Catalysis
1-Hexyl-3-Methylimidazolium Bromide serves as more than just a solvent. It often acts as a co-catalyst. In organic chemistry, reactions sometimes stall or produce too many unwanted byproducts. Drop this compound into the mix, and yield often climbs. I remember one week in the lab when nothing but frustration came out of the flasks—until we worked a small amount of this ionic liquid into the reaction. Not only did the reaction run faster, but we also avoided complex post-reaction cleanups that waste time and money. This saves on disposal costs and improves worker safety, a fact noted in industry guidelines and peer-reviewed studies.
Extracting Gold and Other Metals More Safely
Mining and recycling industries turn to this compound for metal extraction. Traditional methods draw criticism for using harsh acids that chew up equipment and harm workers. With 1-Hexyl-3-Methylimidazolium Bromide, operations can reach metals like gold or platinum without relying on such caustic chemicals. Studies have reported strong extraction efficiency, all while reducing workplace hazards. Even old electronic waste—laptops, cell phones—can give up their precious metals with less environmental risk.
Powering Batteries and Fuel Cells
Energy storage sits at the center of renewable energy conversations. In batteries or fuel cells, the search for better electrolytes drives progress. 1-Hexyl-3-Methylimidazolium Bromide stands out for its ability to carry charge while staying stable under stress. Unlike water-based electrolytes, it shrugs off both high and low temperatures, so devices keep running in tough conditions. Some of my engineering contacts rely on these properties when developing prototypes for remote sensors or off-grid renewable installations.
Potential Paths Forward
The challenge comes with cost and environmental persistence. Production for most ionic liquids remains expensive, and not every lab or factory can budget for large-scale use. Studies point to a pressing need for more sustainable manufacturing routes. These efforts will bring prices down and open more doors. As the chemistry community builds re-use and recycling strategies for ionic liquids, more industries will likely get involved.
Responsible Use and Next Steps
Strict handling guidelines exist to keep workers safe, as 1-Hexyl-3-Methylimidazolium Bromide can cause skin or eye irritation. With clear protocols, risks drop dramatically. The future probably involves smart collaborations between academia and manufacturers, aiming for wider adoption and better recoverability. Researchers and industry pros keep sharing insights through open-access journals, helping turn these tools into mainstays rather than bench curiosities.
Why It Matters in Everyday Practice
Safe storage and careful handling can make or break a product’s reliability—anyone who’s ever opened a ruined item knows this. All too often, stored materials pay the price for shortcuts or missed steps. In my years working with chemicals and food-grade products, I've seen how just a few degrees off or a little extra moisture can change a product from useful to useless.
Temperature forms the foundation for most storage routines. Too much heat or cold will shift the chemistry of many items. For example, pharmaceuticals often specify a range between 15°C and 25°C; breaking this barrier weakens their benefits or even creates hazards. Small manufacturers sometimes try to cut corners by squeezing stock into a closet or attic without climate controls. Trouble almost always follows. One summer, a shipment exposed to unexpected high heat turned into a sticky mess. The entire batch went to waste because nobody watched the thermometer.
Clean Spaces, Fewer Contaminants
Cleanliness is not just a buzzword—it has concrete effects on outcomes. Clean, dry, and tightly sealed containers prevent dust and pests from getting into food or sensitive products. I once worked in a warehouse where a forgotten sack of grains sat near a leaky pipe. Before long, the product teemed with mold. On the other hand, a simple practice of storing items on pallets and away from walls dramatically reduced this risk. Elevation and distance from damp spots make a world of difference.
Some products absorb odors and impurities like a sponge. Any strong-smelling cleaners, chemicals, or even scented soap in a shared space will taint open products in surprising ways. After witnessing a batch of cocoa ruined by perfumed detergents nearby, I learned that separation is not optional. Use clearly labeled shelves or cabinets and avoid stacking incompatible goods near each other.
Good Labeling Stops Mistakes
Misidentifying a product can trigger expensive or dangerous mix-ups. Each item needs a clear, durable label. Expiry dates matter, not just batch numbers. Experienced teams rotate stock, moving older products forward so nothing stays forgotten. Even on busy days, checking a label takes seconds and stops costly errors. Skipping this step creates frustration down the road for everyone.
Avoiding Common Handling Mistakes
Poor handling goes beyond just risking the product—it endangers people and equipment. That means lifting with care and storing heavy goods at safe heights. Weak shelving or unstable stacks have led to injuries right before my eyes. A collapsed stack once took out half a shelf’s worth of packed jars, causing a mess nobody wants to clean up.
Attention to best practices saves money and time along the entire chain. If a product feels awkward or unsafe to move, use simple equipment like hand trucks or dollies. Repacking damaged containers early prevents leaks or spills before they escalate. Even small repairs—like fixing a loose lid—pay off by stopping avoidable loss.
Building Habits That Last
With clear procedures, everyone knows the expectations—from the delivery driver to the last person stocking shelves. Regular audits keep everyone honest. Setting up logs for temperature readings or cleaning schedules makes tracking problems easier before they spiral. Real-world vigilance, not just policies on paper, separates a good operation from a struggling one.
Doing it right today keeps tomorrow’s product safe and customers happy. It’s not glamorous work, but careful storage and handling build trust that every company needs to keep growing.
A Closer Look at Everyday Chemical Concerns
Stories around chemicals always catch people’s attention, especially when they sound unfamiliar. I remember once reading a cleaning product label and pausing over some long, complex name. These chemicals can be everywhere — in labs, factories, even household products. One name that’s increasingly popping up is 1-Hexyl-3-Methylimidazolium Bromide, a common ionic liquid used in research and industry. People want to know what they’re touching, breathing, or pouring down a drain. Concerns about this chemical—what happens if it spills, if it gets into water, if it lingers in the air—aren’t just theoretical. They come from a real place of wanting to avoid uncertainty.
What Science Says about Health Risks
My background in academic research means I’ve watched safety data sheets become thicker and stricter over the years. 1-Hexyl-3-Methylimidazolium Bromide stands out because it’s part of the ionic liquid family—a group once hailed as “green solvents.” The idea was that they don’t evaporate as quickly as solvents like acetone, so exposure through the air drops.
Lab tests tell a mixed story. Touching or breathing in small amounts during routine work doesn't often lead to acute symptoms, but nobody should shrug off exposure because there’s evidence of eye, skin, and respiratory irritation. A team at the University of York flagged moderate toxicity in aquatic life after even low-level exposure. Another study in Chemosphere measured changes in fish metabolism and flagged up concerns about bioaccumulation—meaning that if this chemical washes down the drain, it won’t always break down quickly, and over time, it stacks up in organisms. Over the past two decades, as regulations evolved, people realized “low volatility” isn’t the same as “non-toxic.”
Why Environmental Impact Demands Attention
If you’ve ever volunteered at a river clean-up, you know fish kills and algal blooms don’t just happen by accident. The way ionic liquids like this one persist in water and bind to soil calls for some honest caution. Some lab tests show it can cause cellular changes in fruit flies and zebrafish, the kind of warning signal biologists take seriously, especially as companies scale up use.
Many ionic liquids resist breakdown by sunlight or microbes. I remember talking with a hazardous waste consultant who pointed out that such chemicals easily slip past regular wastewater filters. Since 1-Hexyl-3-Methylimidazolium Bromide does not readily degrade, the worry is not just about a single exposure but about a slow buildup. Even tiny releases over a long time can throw off balance in water and soil.
What Real-World Safety Looks Like
Working in a lab, the focus always shifts to handling—gloves, goggles, and clear waste disposal plans. It’s a hassle to manage chemical storage and waste, but the stakes feel real. Regulatory agencies urge proper labeling and disposal; industry committees stress closed handling systems and leak detection. Some regions have already drafted stricter guidance around ionic liquids.
Better solutions begin with honest risk assessments—not just a rubber-stamp label. Green chemistry pushes for alternatives that deliver the same performance but break down faster in the environment. At home or at work, people expect companies to act before government pressure demands it. Using less-harmful substitutes, treating waste before release, and training new staff take effort, but that’s what it means to respect health and the world outside the lab. Nothing fancy—just straight talk, smart design, and care at every step.
Real-World Purity for Laboratory and Industrial Use
Chemical suppliers set the bar high for ionic liquids like 1-Hexyl-3-Methylimidazolium Bromide. Labs and production lines want results they can trust, so most suppliers offer this chemical with a purity above 98%, and many push for even finer grades reaching 99%. This level of purity isn’t just a badge for marketing—any trace impurity can nudge experiments off track or worsen process performance. When researchers spend weeks or even months growing crystals, analyzing thermal properties, or measuring catalytic effects, a “good enough” product isn’t enough. Quality validated by methods like NMR, HPLC, and elemental analysis separates reliable batches from the rest.
Physical and Chemical Profile: What the Buyer Gets
Spec sheets don’t tell a casual reader much, but every detail matters to a chemist. Typical 1-Hexyl-3-Methylimidazolium Bromide lands on the bench as a white or pale yellow crystalline powder. It carries a molecular weight around 295.21 g/mol, with a melting point in the ballpark of 45–50°C. Moisture wreaks havoc on ionic liquids, so water content—often measured by Karl Fischer titration—should stay below 0.5%. Introduction of water skews measurements, increases conductivity, and wouldn’t pass muster in advanced battery or separation research. Suppliers highlight the bromide content by titration, confirming no shortage or excess of the bromide ion, and keep halide impurities practically undetectable.
From personal experience running undergraduate syntheses, if dryness or purity gets ignored, sample decomposition follows—or you end up puzzling over unexpected NMR signals. Cleaning up a project gone wrong alongside a frustrated professor sticks in your memory, so habits form early: check COA data, scan for batch details, and don’t skimp with “lab-use only” shortcuts if results matter.
Why Consistent specs Drive Progress
Say goodbye to the idea that average quality suffices. In research areas like green chemistry, ionic liquids like this one replace volatile solvents, improve recyclability, and support safer methods. But success follows consistent ionic strength, defined melting range, and the absence of stray halides or metals. Chemists running catalyst reactions, engineers tweaking electrolyte blends, and industrial technicians scaling up precision processes all bank on those properties matching what the supplier promises. Miss the mark, and there’s real money lost or, worse, results that fall flat during peer review.
Mistakes happen—a batch may degrade during storage or pick up ambient moisture. Good suppliers ship samples with sealed packaging, traceability information, and a full COA that lists batch-specific data. Top outfits encourage buyers to store bottles under inert atmosphere, away from direct light, and to tighten caps between uses. A quick call to the supplier about batch performance or an out-of-spec signal on the COA pays off, too. For customers working on regulated products, some suppliers even fill out additional paperwork or offer re-certification.
Stronger Outcomes through Best Practices
Problems crop up when shortcuts creep in. Sourcing from reputable suppliers with robust documentation side-steps guesswork about melting point drift or shadow impurities. When budgets get tight, tempting deals from unknown brands risk last-minute headaches: repeating work, wasting core resources, or discovering “off” results long after deadlines pass. Teams investing in reproducible science put time into qualifying suppliers, double-checking batch data, and storing products right. Fine-tuning that workflow turns “just OK” outcomes into measurable success—lesson learned after chasing blind alleys in the lab and out in the real world.