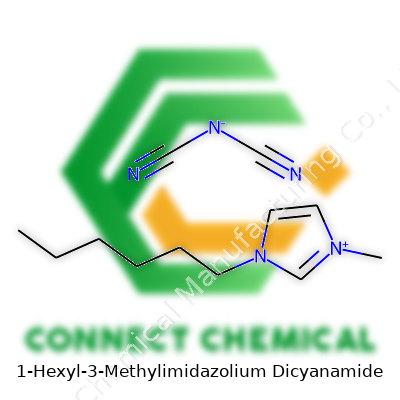
1-Hexyl-3-Methylimidazolium Dicyanamide: An In-Depth Commentary
Historical Development
Chemists first took an interest in ionic liquids decades ago while searching for alternatives to volatile organic solvents. By the late 20th century, researchers assembled a toolkit of imidazolium-based compounds, one of which resulted from combining 1-hexyl-3-methylimidazolium cations with dicyanamide anions. Development ramped up as labs identified special solvent properties in these salts. Academic groups in Europe and Asia raced to publish syntheses and applications, especially since these substances did not evaporate easily and kept their structure at room temperature. The growth in green chemistry and sustainable manufacturing helped push these ionic liquids into broader research corridors.
Product Overview
1-Hexyl-3-methylimidazolium dicyanamide, often known as [HMIM][DCA], has found its way into shelves where chemists reach for solvents that stay liquid under a wide range of conditions. Most bottles contain a clear to light yellow oil with a faint odor. Manufacturers aim for low water content, because too much moisture skews both the physical and chemical properties. Commercial sources often package it in glass or sealed plastic to block air and sunlight. The price reflects labor-intensive purification, but the chemical offers returns in both lab results and safer handling compared to older, more toxic solvents. Most users quickly recognize the value after running side-by-side comparisons with more traditional reagents.
Physical & Chemical Properties
The liquid rarely freezes, sticking close to -20°C to -30°C as its lower limit, while refusing to boil until well past 250°C. Viscosity changes with purity, moving from syrupy to thin with moisture content or degradation. Its density, hovering near 1.05-1.08 g/cm3, feels familiar under pipet or spatula. Conductivity reads high for a solvent—often above 1 mS/cm at room temperature—handy for electrochemical experiments. The dicyanamide anion resists decomposition under mild conditions, but exposure to strong acids or prolonged heat pushes harmful breakdown. Chemists who spend weeks watching reaction outcomes notice subtle changes in reactivity across batches, especially without proper storage.
Technical Specifications & Labeling
Lab-ready [HMIM][DCA] usually comes labeled with its formula (C12H20N6), molecular weight (approximately 248.33 g/mol), and CAS number for traceability. Quality-control data sheets include specs for water content (typically 0.2% or less), halide impurities, and UV-vis absorbance maximas. Storage recommendations emphasize tightly capped containers, shaded from light, ideally in desiccators or dry cabinets. Regulatory codes for shipping warn of potential skin or eye irritation, signaling the need for lab gloves and goggles.
Preparation Method
Synthesis of [HMIM][DCA] often starts with 1-methylimidazole and 1-chlorohexane, choosing a simple alkylation in the presence of base. The resulting chloride salt then goes through metathesis with sodium or potassium dicyanamide, creating the final ionic liquid after extraction, washing, and vacuum drying. Small impurities like hexyl chloride, unreacted imidazole, or residual dicyanamide salts need scrupulous removal to avoid interference in later applications. This extra work slows production and drives costs, but I have seen labs stuck for days running extra purifications after skipping short washes or not monitoring by NMR. Quality at each step saves frustration later on.
Chemical Reactions & Modifications
The compound behaves as a stable non-coordinating solvent for organics, but will not stand up to strong reducing or oxidizing agents. The dicyanamide anion sometimes acts as a ligand, coordinating with transition metals and shifting their redox potentials. Salts like [HMIM][DCA] host catalytic reactions that demand polar, non-volatile media. Some researchers modify the cation tail, swapping out hexyl for butyl or octyl to tune viscosity and hydrophobicity. Replacement of the anion swaps dicyanamide for alternatives, such as bis(trifluoromethylsulfonyl)imide, yielding ionic liquids better suited to different tasks. These structural tweaks let chemists tailor their media without throwing away the imidazolium backbone.
Synonyms & Product Names
Other than the chemically clunky 1-hexyl-3-methylimidazolium dicyanamide, catalogs call this ionic liquid [HMIM][DCA], HMIM DCA, or 1-hexyl-3-methylimidazolium N(CN)2. In global trade, databases log it as CAS 35106-56-4. Brand products sometimes appear under the generic label “imidazolium-based ionic liquids” but the anion and cation distinction matters most for anyone planning to reproduce exact conditions.
Safety & Operational Standards
Most users handle [HMIM][DCA] under chemical hoods, avoiding inhalation or skin exposure. Gloves and eye protection remain standard, with extra caution advised due to the anion’s weak toxicity profile. If spilled, it proves slippery on benches, so clean-up with absorbent pads followed by soap and water becomes routine. Ventilation in storage areas lowers risk as impurities sometimes release irritants when exposed to air. Disposal as non-halogenated solvent waste avoids issues if local rules prohibit pouring down the sink. Local and international regulations, including REACH and OSHA, set documentation benchmarks most chemical facilities follow. Years working with ionic liquids taught me the importance of respecting even “safer” solvents, since chronic exposure can sneak up on careless workers.
Application Area
Chemists found many homes for [HMIM][DCA]. Its non-flammability makes it attractive for battery and supercapacitor electrolytes, where high ionic conductivity meets electrochemical stability. Researchers dissolve cellulose and other biopolymers in it, setting up routes for greener material processing. The compound features in metal extraction, carbon dioxide capture, and catalytic reactions where persistent solvents boost yields and minimize waste. Spectroscopists appreciate its broad solvency range—aromatics, inorganics, and even biological samples mix smoothly in its grip. Chemical engineers scale up these results, devising systems for continuous flow reactions that shun more hazardous aromatic or chlorinated solvents. It proves versatile, with key breakthroughs driven by teams exploring outside textbook recipes.
Research & Development
The scene around [HMIM][DCA] thrived as funding for green chemistry swelled in the 2000s. Dozens of papers each year detail new catalytic applications, solvent recycling schemes, and hybrid electrolytes. Tech startups tinker with ionic liquid-based separation processes or energy storage formulations. Collaborations between academia and industry focus on process intensification, aiming to squeeze more efficiency from every liter used. As patents build up, manufacturers tweak the purity, packaging, and stability to serve different research and industrial audiences. Conferences discussing ionic liquids always highlight how small molecular changes reveal surprising behavior, which drives ongoing inquiry.
Toxicity Research
Questions around toxicity hang over any new chemical class. Early hopes for ionic liquid safety faded after some lab tests found imidazolium cations, including 1-hexyl-3-methylimidazolium, disrupt aquatic life and slow microbial degradation in wastewater. The dicyanamide anion fares a bit better, but chronic exposure data remains sparse. Strictly controlled studies in rodents and cell cultures point to eye and skin irritation, and there’s ongoing debate about potential mutagenicity after long-term contact. Environmental chemists press for robust data before these compounds go mainstream, and responsible labs track waste streams closely. Safe disposal and containment remain essential, and work continues on redesigning the molecules for improved biocompatibility and easier breakdown over time.
Future Prospects
Scientists drive the field by pairing ionic liquids like [HMIM][DCA] with sustainability targets, energy challenges, and advanced composites. The non-volatile nature gives them a green luster, but wide adoption waits for breakthroughs in production costs and clearer answers on health risks. Fundamental research keeps discovering new uses, from antifouling coatings to pharmaceuticals, testing unique solvent effects in otherwise tricky syntheses. Startups pitch these ionic liquids as solutions to current chemical problems, sparking investments and new ventures. As legislation tightens around emissions and safety, the pressure mounts on chemists to deliver both performance and responsibility. Hands-on experience shows that while ionic liquids solve a lot of old problems, their complexity means new ones often hide just around the corner.
Beyond the Lab: Why Chemists Look West
Lab technicians and materials scientists have always hunted for alternatives to harsh chemicals. About twenty years back, 1-Hexyl-3-methylimidazolium dicyanamide appeared on the scene, and people in research labs realized it was more than another jar on the shelf. Chemists like it because it behaves like an ionic liquid—it flows at room temperature, doesn’t give off nasty fumes, and keeps things stable even when things get hot. The main story here is about replacing dangerous stuff with something that works better and handles easier.
Chemical Extraction in the Real World
Metals are part of modern life, from batteries to wind turbines. Extracting these metals poses real environmental and worker-safety issues. Traditional methods often rely on petroleum-derived solvents or acids that corrode both equipment and lungs. 1-Hexyl-3-methylimidazolium dicyanamide can offer a safer path. In practice, its unique make-up lets it grab selective metals during extraction, making cleanup less stressful. In copper mining, for example, it helps pull out copper ions without dissolving everything else in sight. It’s not only good news for workers but also slashes costs for environmental compliance.
Green Chemistry in Industry
Chemical manufacturing stands under huge pressure to cut emissions and waste. This ionic liquid supports greener approaches. Take cellulose processing. Usually, pulping wood needs a tough chemical like sodium hydroxide. If you swap in this newer solvent, you break down cellulose much faster, with less waste and no sharp odors. Textile plants experimenting with it find their air cleaner and their wastewater easier to treat. It’s not pie-in-the-sky; BASF and Dow have invested in this research, showing just how mainstream this author believes it will become.
Batteries, Electronics, and Energy Storage
Inside batteries, safety and lifespan depend on the tiniest details. Electrolytes drive ion movement, and a leak or fire spells trouble. This liquid doesn’t catch fire under battery conditions. That means safer electric cars and laptops. Plus, it won’t break down after a few charge cycles. Companies like Samsung and LG spin off research papers showing longer battery life when they use this stuff as the electrolyte solvent. For the consumer, that translates into phones that don’t overheat and electric cars that survive a few extra winters.
Catalysis: Pushing Industry Forward
Factories depend on tiny catalysts to speed up chemical reactions. This material acts as both a solvent and a co-catalyst. Petroleum refining, drug synthesis, plastics—these businesses gain by cutting waste production and speeding up reaction times. The dicyanamide part kicks in as an active participant, not just a bystander. Process engineers cut down on solvent swaps or complicated extra washing steps. That’s money saved on the factory floor and less headache meeting regulatory audits.
The Hurdles and the Road Ahead
Some people in research circles raise flags about cost and recycling of ionic liquids. Prices have dropped over the last decade, but wide adoption still bumps against budgets in small companies. Chemical makers now look at how to reclaim and clean these liquids after use. Companies stack reactors with filters and distillation columns, catching and reusing every drop they can. Some universities are even exploring ways to make these molecules from plant oils rather than oil barrels, hoping to turn a laboratory marvel into something sustainable—no greenwashing, just good science leading to safer industry.
Why Chemical Caution Matters
Handling chemicals with long names and serious properties doesn’t just mean being careful—it draws on real knowledge, respect, and using the right gear. 1-Hexyl-3-Methylimidazolium Dicyanamide, commonly used in research labs, offers interesting options for chemists because of its low volatility and usage as an ionic liquid. But unlike water or sugar, these features don’t mean you can get careless during handling or disposal. There’s a health reason behind every step people take in a lab, and this one is no different.
Direct Contact: Risks You Can’t Ignore
Chemicals with cyano groups, like dicyanamide ions, demand respect. Inhaling, swallowing, or even having the solution touch bare skin can trigger harmful reactions. Studies on similar compounds show possible toxicity. A friend of mine who worked with hydrogen cyanide still remembers the skin tingling—never took his gloves off near the stuff again. For this compound, any splash or contact stays with you, literally and figuratively.
A typical lab coat isn’t enough if you expect splashes; always add chemical-resistant gloves and goggles. Ordinary latex gloves break down too easily, so nitrile stands out as the better pick. Good goggles—not just glasses—seal tightly, buffering your eyes from both vapors and accidental splashes. Don’t overlook the simple act of tying back loose hair and pushing up sleeves. Any stray surface offers a route for contact, and skin absorbs more than most people think.
Handling and Storage: Don’t Trust Your Memory
Ask anyone who’s mislaid a sample: one lapse leads to contamination or worse. 1-Hexyl-3-Methylimidazolium Dicyanamide needs storage in well-sealed, labeled containers, far from food, acids, or oxidizers. Sturdy shelving, not above head height, matters in case you bump a bottle. Walk into any lab worth its salt, and you’ll see chemical storage arranged with accidental mixing in mind.
Ventilation also makes a difference. Odorless doesn’t mean harmless, as some ionic liquids release fumes you can’t smell or spot. Fume hoods keep vapors moving away from you, not into your lungs. Quickly capping bottles after use and wiping down benches isn’t just habit—it reduces risk for everyone, not just you.
Spills: Quick Response Beats Regrets
Nobody forgets the first time they spill something hot, sharp, or toxic. Training for chemical spills still means focusing on immediate action. Absorbent pads and neutralizing agents need to be close at hand—scrambling to find them wastes time. Once a spill happens, the area needs attention right away. People who sweep up glass or liquid with bare hands without thinking about the risk often end up with more trouble than they bargained for. Clear up everything with gloves on, including tools and doorknobs.
Disposal follows the rules local authorities and the EPA provide. Down the drain isn’t an option for dicyanamide compounds. Sealed hazardous waste containers and accurate logging cut down on long-term risks. Teaching newer lab members these steps saves time, money, and a lot of headaches.
Better Habits Start Here
Many labs treat safety as an ongoing lesson, not a checklist. From my own time in research, swapping stories about close calls set habits in ways signs on the wall never did. Investing in personal protective equipment and clear written protocols means nobody has to learn lessons the hard way. The peace of mind that follows good habits stands out more than any warning label ever could.
Breaking Down the Formula
Chemistry picks a curious way of naming things. With 1-Hexyl-3-Methylimidazolium Dicyanamide, you get a long name and an even longer story. You’ll see it written as C12H22N6 for the formula, which brings to mind a molecule shaped by real teamwork. To understand what this material looks like at the atomic level, you have to pull its name apart.
The main component is the imidazolium cation. It comes from an imidazole ring, which is a five-membered ring with nitrogens at positions 1 and 3. Methyl sticks to one nitrogen, while a hexyl group holds tight to the other. Making sense of it in two dimensions, you get a core that’s flat and aromatic, with one tail short and the other long and greasy. The positive charge stays put on the ring, mostly on the nitrogens.
Then you have the dicyanamide anion (N(CN)2-). It’s built from three nitrogens, two of which bridge to their own carbon, and each carbon stretches off to a triple-bonded nitrogen atom. Think of it as a central N taking both arms of C≡N, making a pointy, linear ion that balances the positive charge from the imidazolium.
Why This Structure Matters
Nobody just wakes up and makes a molecule like this for fun. In the lab and in industry, folks started mixing these specific chemical features because they wanted a new breed of solvent. These so-called “ionic liquids” aim to cut down on toxic, flammable, or volatile organic solvents. Here, the hexyl tail means the cation offers some oil-friendly, hydrophobic character, while the dicyanamide brings in charge without sticking everything together like glue.
This combination doesn’t evaporate easily, so you get a liquid that lingers at room temperature without throwing fumes. The molecular tinkering lets people use these substances not just as solvents but also in batteries, catalysts, and polymers. The flat imidazole and pointy dicyanamide connect—sometimes weakly, sometimes tighter—allowing for chemical flexibility that older solvents simply couldn’t provide.
Real-World Value and Responsible Use
Studying and using 1-Hexyl-3-Methylimidazolium Dicyanamide worked out for many researchers wanting safer, greener processes. Still, not every “green” label tells the whole story. The dicyanamide group contains cyano units, which carry some real risks if not handled carefully. Even if the main liquid resists evaporation, toxic breakdown products could appear if it gets too hot or sits in the wrong place.
Careful handling and proper disposal matter just as much with new chemicals as with older ones. The growing list of ionic liquids means more testing for toxicity, environmental impact, and safe design. Regulators and scientists don’t always move at the same speed, but curiosity and caution go hand in hand during development. Labs need clear safety sheets and real environmental reviews, not just enthusiastic press releases.
People in the field keep finding ways to swap in less hazardous groups if any real concern turns up, a healthy check that reminds us innovation should respect both safety and sustainability. Responsible research and transparent sharing of data help make progress science can stand behind. This story, like the molecule itself, relies on the way all its parts connect.
Everyday Lab Realities and Chemical Storage
Chemists who regularly handle ionic liquids like 1-hexyl-3-methylimidazolium dicyanamide know these substances don’t behave quite like the common solvents you find tucked away on a classroom shelf. This material’s unique qualities, such as its low melting point and high thermal stability, can lull researchers into a false sense of security. Complacency, in my own experience, always opens the door to accidents or spoiled work. For many, a university glove box or shared chemical cabinet might serve as the first line of defense. Keeping chemicals safe doesn’t just protect equipment — it ensures projects don’t get derailed by contamination or unexpected reactions.
The Role of Moisture and Air Quality
1-Hexyl-3-methylimidazolium dicyanamide absorbs water from the air like a magnet draws metal shavings. This hydrophilic streak has wrecked more than one batch in my time, especially during humid summer spells. Once, a bottle left uncapped for only a few minutes picked up enough moisture to ruin the delicacy needed for a sensitive electrochemistry experiment. Storing this material in a tightly sealed container — a glass stopper beats a plastic cap most times — reduces this nightmare. Keeping it in a desiccator, where humidity stays low, adds another layer of defense. These strategies reflect what experts at the American Chemical Society and similar bodies recommend. It’s always a small effort to replace silica gel or recharge a desiccant, but the payoff in chemical purity has saved my lab group countless hours and headaches.
Temperature Control: More Than Just a Number
Cool storage keeps 1-hexyl-3-methylimidazolium dicyanamide in its sweet spot. Most manufacturers say room temperature suffices, but temperatures swing fast in older buildings. I remember a time when a thermostat malfunctioned and the lab soared above 30°C all weekend; more volatile chemicals vented off, and the ionic liquids visibly changed consistency. So, cool and constant temperatures bring peace of mind, even if it means stashing bottles away from windows or heat sources. For anyone working in shared labs, where sunlight spills through glass and heats up benchtops, vigilance matters. Chemical Safety Board reports often cite “unlabeled” or “misplaced” containers — preventable with careful planning.
Protecting Against Light and Chemical Reaction
Bright light may not break down every chemical, but it increases the risk for photodecomposition. For ionic liquids, opaque bottles or even wrapping containers in aluminum foil can block damaging UV rays. I used to underestimate this precaution, until a colleague showed me discolored samples from long-term shelf exposure. Discoloration signals possible by-products, which can spoil analytical results or throw off synthesis yields. It’s tempting to cut corners, but storing chemicals away from direct sunlight helps preserve quality, no matter the lab's size or budget.
Clear Labeling and Organization
Any bottle deserves a legible, waterproof label. I’ve seen too many near-misses caused by faded ink or cryptic abbreviations — mistakes that can have serious consequences, especially given the toxicity of dicyanamide salts if mishandled. Grouping similar chemicals, keeping new and old stocks separate, and up-to-date safety data sheets within reach all pay dividends. These habits don’t develop instantly, but they foster a culture of safety that protects both people and research output.
Learning from Experience
Practical knowledge grows with every mistake and every careful correction. For students and experienced chemists alike, the best storage practices — sealed glass, low humidity, consistent temperature, and limited light — are not empty rituals, but the backbone of consistent experimental results. Sharing experiences and keeping up with new research help everyone make safer, smarter choices in the lab.
Not Just Another Ionic Liquid
Lab benches hold plenty of interesting chemicals, but 1-hexyl-3-methylimidazolium dicyanamide (often named [HMIM][DCA]) grabs attention for a reason. It shows up as a clear to pale yellow liquid with no strong smell. Unlike water or common solvents, this compound barely evaporates at room temperature. Glassware comes away without traces of wetness, showing just how little the vapor escapes into the air. That’s not a small deal for anyone who dislikes fume hoods or constant solvent loss during reactions.
Physical Properties That Matter
Pour it, and you’ll notice it flows less freely than water but still spreads easily. Its density hovers around 1.0 g/cm³, just like water, which makes it simple to measure and combine in test tubes or flasks. The melting point doesn’t matter much unless you’re freezing your lab, since it’s below room temperature, keeping it liquid under most storage conditions. As for boiling, it survives serious heat, so routine lab heating won’t send it billowing away.
People worried about spills or fires feel some relief because 1-hexyl-3-methylimidazolium dicyanamide barely catches fire. High thermal stability helps–it stands up to temperatures over 300°C before breaking down, which beats standard solvents like acetone or ethanol. Water dissolves it with some effort, but it’s more at home with other organic liquids. Not much will dissolve plastics or rubber, but this ionic liquid sometimes manages to soften seals after long exposure.
Chemical Side: Why the Structure Stands Out
Those two dicyanamide groups packed into the molecule bring real versatility. The dicyanamide anion doesn’t react easily, so it won’t jump into side reactions and mess up careful experiments. On the positive side sits the imidazolium cation, which makes the compound ionic—but not in the usual sense of salts like sodium chloride. Instead, the charge spreads through the molecule and helps keep reactions in check. Greater ionic strength boosts the solubility of metals and other salts in the mix, which researchers like for greener chemistry.
It surprises many that such salts barely conduct electricity compared to sodium chloride solutions, but 1-hexyl-3-methylimidazolium dicyanamide still gets used as an electrolyte in batteries. That’s a trade-off: slower ion movement, but less risk of leakage or explosion. It won’t dissolve everything, but it can dissolve a wider range of chemicals than plain water or most oils. For extra bonus points, it doesn’t go sour or stink up the room over time, which is a constant worry with organic solvents like THF or ether.
Why This Matters in the Lab—and Beyond
Switching from volatile organic solvents to ionic liquids brings health benefits. I remember working with acetone all day, ending up with dry hands and a headache from fumes. After switching to ionic liquids like [HMIM][DCA], the change felt immediate: fewer headaches, less worry about spills, and no more burned-out sinuses from evaporation. Scientific literature backs this up—ionic liquids emit less vapor, making indoor air safer. Problems linger—waste handling needs strict protocols. Things don’t break down easily. Labs using more green solvents like [HMIM][DCA] must set up ways to collect and recycle their waste because landfills can’t handle persistent salts forever.
Pushing toward safer chemistry means keeping an eye on physical and chemical features like volatility, reactivity, and toxicity. Facts matter. 1-hexyl-3-methylimidazolium dicyanamide brings real improvements over traditional choices, but no one should treat it as a miracle fix. Balancing the unique benefits with proper safety steps opens doors for greener, more sustainable science. If the toughest part means double-checking disposal bins and reading a few more safety sheets, that feels worth it.