1-Hexyl-3-Methylimidazolium Hexafluoroantimonate: A Commentary on Its Scientific Journey and Future
Historical Development
Back when ionic liquids were making their way from labs to actual chemical processes, 1-Hexyl-3-Methylimidazolium Hexafluoroantimonate turned plenty of heads for good reason. Chemists searching for alternatives to traditional solvents picked up on it in the late 1990s, riding on the wave of “green chemistry.” They hadn’t seen many salts like this one, which stayed liquid at room temperature and dodged the volatility problems regular organics kept tossing up. Early work with imidazolium-based ionic liquids often focused on tailoring the cation and anion to nail down a sweet spot between stability, conductivity, and handling. Researchers found hexafluoroantimonate anions gave these fluids some extra thermal and electrochemical backbone compared to run-of-the-mill halides or tetrafluoroborate salts. Fast forward a bit, and this compound starts showing up in academic papers as a star player in electrochemistry, catalysis, and separation science.
Product Overview
1-Hexyl-3-Methylimidazolium Hexafluoroantimonate does not look like your average salt. At room temperature, it’s a viscous, colorless to pale-yellow liquid that refuses to evaporate under normal conditions. Thanks to its imidazolium ring tagged with a hexyl group, it handles hydrophobic and hydrophilic molecules without fuss, giving it an edge over old-school solvents. Most sellers package it in tightly sealed amber glass bottles, because the hexafluoroantimonate part gets jittery around light and moisture; the last thing anyone wants is slow hydrolysis tearing apart the anion and spoiling a costly batch. Ingredient lists are refreshingly short here—no hidden additives, just pure ionic liquid designed for use right out of the bottle or after a quick vacuum drying session.
Physical & Chemical Properties
Every scientist I’ve met who used this ionic liquid talks about its density—that’s one way you know you’re handling something unusual. Density clocks in around 1.2 to 1.4 grams per cubic centimeter, depending on impurities and temperature. High polarity and minimal vapor pressure make it a fixture in glovebox work, especially for electrochemists who can’t risk water or volatile organics mucking up measurements. Its thermal window runs wide, typically staying stable from -20°C up to 200°C. It mixes with other polar solvents like DMSO or acetonitrile, but water starts to break down the anion, so you’ve got to keep it dry. Electric conductivity ranks high compared to many ionic liquids, which feeds its popularity in batteries and capacitor studies.
Technical Specifications & Labeling
Quality control comes down to purity—research-grade bottles promise at least 98% purity, with water content measured down around 500 ppm or less. Every vial ships labeled with CAS number, lot code, and storage recommendations (usually under nitrogen, away from light, inside desiccators). The bottle’s safety label notes its hydrolysis risks: when exposed to moisture, it can release toxic antimony compounds and HF, so protective gloves and goggles are standard. You’ll run into many different ways companies abbreviate the name; sometimes just [HMIM][SbF₆], or the full 1-hexyl-3-methylimidazolium hexafluoroantimonate, to cover all customs and regulatory requirements.
Preparation Method
Most bench-scale syntheses start with 1-methylimidazole and 1-chlorohexane, which react to form the precursor salt 1-hexyl-3-methylimidazolium chloride. This intermediate gets purified—usually through repeated washing and extraction—then converted by metathesis, reacting the chloride salt with silver hexafluoroantimonate in dry, oxygen-free conditions. This route grew popular because it minimizes contamination by leftover halides or water. After filtration removes insoluble silver chloride, careful evaporation brings out the target ionic liquid. In my own experience, working under dry nitrogen and chilling the reagents helped cut down on decomposition and kept yields high.
Chemical Reactions & Modifications
One of the things I respect about this compound is its resilience under tough synthetic conditions. Chemists use it to dissolve organometallic intermediates or run tough acid-catalyzed reactions, where conventional solvents would either catch fire or contaminate the catalyst. Its imidazolium core can be swapped or tweaked for longer alkyl chains, giving rise to variants with different melting points or solubility profiles. On the flip side, the hexafluoroantimonate anion can get nudged out under harsh acid treatment or in the presence of strong nucleophiles, leading to partial breakdown and complicated side products. Adding functional groups to the ring structure yields custom ionic liquids for separations or extra resistance to electrochemical degradation—a field where every tweak opens new application doors.
Synonyms & Product Names
In the wild, expect to see abbreviations like HMIM+ SbF6– or 1-Hexyl-3-methylimidazolium hexafluoroantimonate, sometimes dropped to just “hexafluoroantimonate IL” in older publications. Some catalogues throw “IL-50” or similar codes into the mix depending on the cation length. For clarity, most chemical safety databases stick with the full name and CAS number, since shorthand can trip up international shipping or regulatory reviews.
Safety & Operational Standards
I always keep an extra layer of respect for this category of liquids. Handling this stuff outside a glovebox is inviting risk—exposure to moisture releases hydrofluoric acid and antimony compounds, both of which call for medical attention at the slightest sign of contact or inhalation. Fume hoods, powder-free nitrile gloves, and splash-proof goggles are minimum requirements. Disposal goes through hazardous waste channels; this compound can’t hit the drain or regular trash, given the long-term toxicity of antimony and the bite of released fluoride. Emergency protocols in most labs include calcium gluconate and spill kits near all ionic liquid benches.
Application Area
Real-world demand for ionic liquids like this one rises out of their surprising ability to stay stable across a messy mix of reaction conditions. In research, you’ll spot this compound in green chemistry showing off its zero vapor pressure and low flammability. Electrochemical devices benefit from its wide electrochemical window, letting innovators stretch the limits of battery electrolytes and supercapacitors, especially where thermal runaways have trashed earlier prototypes. Catalysis labs use it to speed up reactions that traditional solvents can’t touch, including difficult Friedel-Crafts or metal-catalyzed couplings. Beyond the lab, there’s strong push in separation sciences; the substance dissolves organic, organometallic, and even some polymeric materials that refuse to budge in most media.
Research & Development
Funding agencies ramp up support for work with ionic liquids every year, but this one pulls in steady grants thanks to its unique antimony anion. Current research runs the gamut from designing custom solvation shells for protein studies to optimizing electrochemical sensors that track environmental toxins. Several universities pour effort into testing new derivatives, hoping to amplify ionic conductivity while cutting down the toxicity that rides along with the heavy metal anion. Scale-up isn’t always straightforward—the costs of hexafluoroantimonic acid and strict handling standards slow industrial adoption—but growing evidence from battery labs and green tech start-ups speaks to the underlying value.
Toxicity Research
Toxicology groups have a love-hate relationship with the hexafluoroantimonate family. Animal and cell studies point to the antimony component as a long-term hazard. Chronic exposure links up with respiratory and cardiovascular risks, a pattern matching what scientists see for other antimony salts. Acute exposures to vapor or mist can chew up tissues with released hydrofluoric acid; the resulting skin, eye, and lung damage take weeks to heal. Regulatory agencies in Europe keep ionic liquids containing antimony under tight watch, mandating closed systems and routine monitoring of air and surface contamination. Labs with any track record of near-misses now double up personal protective gear and invest in regular medical exams for their handling crew.
Future Prospects
Everyone I talk to in advanced materials circles expects the next decade to see more targeted design of ionic liquids—balancing cutting-edge properties while dialing back on environmental baggage. For 1-Hexyl-3-Methylimidazolium Hexafluoroantimonate, the most promising future sits in high-performance energy storage, precision catalysis, and complex chemical separations that demand solvent power and stability regular organics can’t offer. Ongoing work tackles toxicity with substituted anions and greener synthesis methods—moving toward safer, more scalable versions. Every successful tweak here edges technologies like safe batteries, recyclable polymers, and efficient chemical sensors that much closer to our shelves, while keeping the risks from antimony and fluoride front and center in future policy debates and lab safety routines.
Unlocking the Power of Modern Chemistry
Most people don’t hear about substances like 1-hexyl-3-methylimidazolium hexafluoroantimonate outside of academic journals or chemical supply houses, but research labs and high-tech industries know this compound by its unique traits. With a tongue-twisting name, this liquid offers some clever solutions to real-world problems where ordinary solvents and salts fall short.
What Makes This Liquid Tick?
Classic solvents bring flammability, harsh smells, and health hazards to the table. Chemists have been hunting for alternatives that don’t light up or evaporate so easily. Here, ionic liquids step in. This particular one—often shortened inside labs to its initialism, HMIM SbF6—stays liquid at room temperature, doesn’t give off fumes, and handles a broad range of tasks. The trick lies in its pairing of a chunky organic cation with a heavy, stable anion that keeps everything stable and unreactive with air and water.
Changing the Game in Electrochemistry
Most folks don’t think about what’s needed to build the batteries in their phones or the sensors that run medical equipment. Traditional electrolytes—stuff that ferries charges in batteries—expose users and manufacturers to toxic or flammable chemicals. HMIM SbF6 brings much lower risk in terms of fire and offers a wide voltage range, letting engineers push batteries harder without meltdown. Research since the early 2000s shows ionic liquids like this one improve both energy storage and the lifetime of lithium-ion and next-generation battery prototypes.
A Safer Choice for Complex Reactions
Organic synthesis—making new molecules for medicines or advanced materials—often gets messy, especially if the solvents get in the way of delicate chemical steps. HMIM SbF6 offers a stable backdrop where molecules can break and form bonds without side reactions. Its nonvolatile nature means less exposure for workers and reduces the headaches of cleaning up after solvents that evaporate or catch fire easily.
Committed to Cleaner Industrial Processes
Modern manufacturing looks hard at every step in a process, hunting for greener alternatives to longtime polluters. More industries line up to test ionic liquids in place of hazardous substances for cleaning, separating, or purifying chemicals. HMIM SbF6’s chemical stability and low vapor pressure match the call for sustainable solutions that don’t risk air quality or worker health.
What Lies Ahead?
Cost still crowds out widespread use outside of research or high-end materials. These ionic liquids demand careful handling during both production and disposal, as their tough molecular structure resists breaking down in the environment. The push for green chemistry keeps driving researchers toward safer, cheaper versions without toxic byproducts.
Anyone working in science or engineering today faces a tradeoff—picking materials that push performance higher without tripping over safety or environmental rules. Compounds like HMIM SbF6 mark a step forward. As manufacturing changes and batteries, sensors, or drug-makers keep raising their standards, the appetite for smarter, safer solvents only grows. Scientists at the lab bench and on the factory floor both want the same result: better chemistry that keeps people and the environment out of harm’s way.
References: - Rogers, R.D., Seddon, K.R., “Ionic Liquids—Solvents of the Future?” Science, 2003.- Welton, T., “Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis,” Chemical Reviews, 1999.- U.S. Department of Energy, “Ionic Liquids in Energy Storage Applications.”
Recognizing Real Risks in the Lab
Anybody who spends time in a research lab knows chemicals like 1-Hexyl-3-methylimidazolium hexafluoroantimonate bring unique hazards along with their usefulness. Most folks don’t come across ionic liquids every day, but this substance, with its tongue-twisting name, turns up in batteries, catalysis, and some advanced material research. You can’t just open the bottle and have at it. Exposure to this compound can create serious problems for your skin, lungs, and eyes. Though less volatile than other chemicals in the lab, ignoring its hazards carries heavy costs.
Direct Contact Isn’t a Minor Issue
I learned early that even clear liquids that “don’t look dangerous” can bring skin burns or cause havoc to your eyes. Spilled ionic liquids like this one soak into gloves and clothing if you grab the wrong material. I always reach for heavy-duty nitrile gloves, checking they’re rated for chemical resistance. Eyewash stations have saved my colleagues more than once, but prevention wins every time. Lab coats, goggles, and face shields block splashes and keep you from scrambling for first aid after an accident.
Inhaling Fumes: More Harmful Than You Think
Just because a substance doesn’t stink or make your eyes water doesn’t mean it’s safe to breathe. Hexafluoroantimonate salts can release toxic vapors, especially in poorly ventilated spaces or when mixing with acids. I never trust open air alone—if I’m measuring this compound, I make sure the fume hood’s sash is pulled down and that exhaust fans run full tilt. A fit-tested respirator is more than just belt-and-suspenders thinking some days. When you consider antimony compounds can build up in your body over time, you quickly realize shortcuts aren’t worth the risk.
Labeling, Storage, and Housekeeping: Small Details, Big Impact
Once you get comfortable with a chemical, it’s easy to start treating the bottle like a jug of vinegar. That habit leads to trouble. I always double-check the label, writing the full name out—not just the abbreviation, since visitors or newcomers may not know what’s hiding inside. Keeping the bottle tightly sealed, dry, and away from acids or strong bases is about respecting both the chemical and shared lab space. Just a pinprick leak or a layer of white dust on a benchtop means it’s time to break out the spill kit, not “wipe it up and move on.”
Training and Planning: The Foundation for Lab Safety
Proper handling comes down to building good habits and following set procedures, not just reading safety data sheets and forgetting them. I’ve seen safety culture improve when teams walk through a risk assessment together—talking out loud about what could go wrong if a beaker tips, or if an experiment runs overnight. Emergency plans, posted phone numbers, and real drills save time when minutes count. Respect for the substance grows once you realize mistakes ripple out: a burned hand or contaminated air doesn’t stay personal for long.
Stronger Policies, Safer Outcomes
Administrators shape safety culture. They make sure personal protective equipment fits, that fume hoods get serviced, and that training sessions stay mandatory, not optional. Investing here saves far more downstream than any cleanup or workers’ compensation claim. Labs that share stories of close calls, not just accidents, build trust and caution where both matter most. Real accountability—through regular audits and open feedback—keeps everyone sharp, not just the new grad students.
Understanding the Risks and Realities
Walking through any chemical storage room, you can spot a few substances that need more than a plastic jug and a dusty shelf. 1-Hexyl-3-Methylimidazolium Hexafluoroantimonate is one of those. It’s an ionic liquid, used for catalysis, electrochemistry, and some cutting-edge battery research. The catch with this compound isn’t just about melting points or solubility—a little mishap can turn a handy tool into a laboratory hazard.
Moisture and Air: Hidden Enemies
1-Hexyl-3-Methylimidazolium Hexafluoroantimonate reacts easily with water. That’s not something you want drifting into your project or breathing into the room. Water in the air may lead to decomposition, corrosion, and release of HF (hydrogen fluoride). Every time someone claims humidity in a storage room “won’t hurt,” think about slow leaks or half-closed jars—permanent reminders of what happens when routine fails.
Finding Strong Barriers to Exposure
Proper storage containers actually make a huge difference here. Tight-sealed glass bottles with PTFE lids or specialized fluoropolymer coatings block the steady crawl of moisture and oxygen. It never feels glamorous, handling vials and lining up flasks, but small habits protect everyone. It pays to double-check labels and date them. By the time you sniff a whiff of acid or see jar labels peeling, it’s already too late.
No Room for Half-Measures
Shelving these compounds away from direct sunlight stops heat from slowly breaking down the structure. Keeping temperatures cool matters—not ice-cold, but steady and away from furnace blasts or sunbeams. A regular chemical fridge usually works, as long as it isn’t the same one where lunch leftovers tempt fate. Light-sensitive containers or storage cabinets extend shelf life and reduce accidents.
Eyes Wide Open: Lab Design and Culture
Looking back at three years managing a university stockroom, the safest labs weren’t the ones with the newest gear—they were the ones where every assistant owned their own routine. Every morning, a once-over on container seals. Every evening, rotating older stock forward. Emergency spill kits stood in clear view. People wrote on logs by hand, which slowed things down but left a real trail. Oversight and common sense beat cutting-edge technology in almost every case.
Supporting Facts from the Field
Numerous hazardous substance databases point out long-term storage risks for hexafluoroantimonate salts. The release of HF is not just a footnote: hospitals in urban research centers have tracked burns and respiratory injuries linked to improper chemical storage. With increasing use in green chemistry and energy labs, the stakes get higher. Regulatory bodies and manufacturers both recommend storing such chemicals dry and cool, inside fume hoods or specialized ventilated cabinets, away from acids, bases, or incompatible fluorinated materials.
Everyday Solutions, Not Just Lab Myths
Accountability runs on small details: regular inventory, double labeling, and training every new researcher. Regularly check for color or texture changes—don’t brush off anything that looks or smells “off.” Upgrade old cabinets and bins when signs of rot or warping appear. Avoid collecting more chemical than a lab can responsibly use in one year.Manufacturers can pitch in with better packaging and more visible hazard warnings, but most safety depends on people in the room. Sharing real-life mishaps, not just textbook rules, reminds everyone what’s at stake. Storing 1-Hexyl-3-Methylimidazolium Hexafluoroantimonate correctly isn’t just about following a checklist—it’s about keeping science smart, safe, and sustainable.
Digging Into the Chemistry
Chemistry classrooms and labs love tricky questions around solubility. Tossing a tongue-twister like 1-hexyl-3-methylimidazolium hexafluoroantimonate into the mix probably sends a shudder through even seasoned scientists. It’s an ionic liquid, and folks often ask if it dissolves in water. Ionic liquids come in all shapes, sizes, and solubility behaviors, so generalizing rarely pays off.
My curiosity for ionic liquids began in graduate school, where eco-friendly solvents attracted attention. Ionic liquids, compared with many traditional solvents, don’t evaporate easily and usually don’t have a strong odor. That makes them handy in labs that want to avoid fumes. Still, what really matters for handling, applications, and safety is knowing what happens when they hit water. Unlike ethanol or acetone, which mix without fuss, the big cations and bulky anions in room-temperature ionic liquids can act very differently.
Water Solubility: Fact Versus Assumption
Look closely at 1-hexyl-3-methylimidazolium hexafluoroantimonate. It couples an organic cation with a hefty, hydrophobic anion (hexafluoroantimonate). Textbooks and company technical sheets note that hexafluoroantimonate-based salts trend toward lower water solubility. One reason is the large anion: it doesn’t interact smoothly with water molecules the way smaller, high-charge-density ions do.
Experiments back this up. I remember shaking up a sample of a similar ionic liquid with water, waiting for a clear layer to form, and seeing two distinct phases. Not much mixing took place. Published research backs these hands-on experiences by listing most hexafluoroantimonate ionic liquids as sparingly soluble to nearly insoluble in water. Adding more hydrophobic groups—like the hexyl chain here—pushes the compound even further toward water rejection.
Why Solubility Really Matters
Some might wonder why anyone should care if this particular ionic liquid dissolves in water. It makes a big difference for environmental safety and industrial use. If a chemical dissolves in water, it can move far and wide in soil or rivers. That adds risk. When a compound resists dissolution, it tends to stay put, but spills can leave sticky residues that are hard to clean.
In my industrial consulting days, companies planned processes around the chemicals’ behaviors. Difficult-to-remove residues meant more cleaning steps and costs. Trying to dispose of a hydrophobic ionic liquid demanded creative waste solutions and could raise eyebrows with regulators, since standard water-based cleanup methods didn’t budge the stuff.
Building Safer, Smarter Chemistry
Green chemistry grows from tough questions like these. If a solvent or additive is needed, folks should weigh not just performance but cleanup and environmental impact too. Ionic liquids look clever as solvents for tricky reactions, but manufacturers should pick ones that balance performance, cost, and safety. If 1-hexyl-3-methylimidazolium hexafluoroantimonate doesn’t blend into water, then its use outside a controlled environment or in processes where leaks are possible requires thoughtful planning.
Switching to more water-friendly or biodegradable options can lower risks and ease regulators’ concerns. Routine lab work I'm familiar with includes a step called a "bench test": mixing a sample in water and seeing what happens. Simple tests like this, plus readable and open data sharing, help scientists, companies, and the public understand what lurks behind complex chemical names.
Looking Forward
Responsible science means sharing clear information on every step of a chemical’s journey—from the flask to the wastewater treatment plant. The less mystery around these compounds, the better for lab safety, environmental protection, and public trust. That starts with a plain answer: No—1-hexyl-3-methylimidazolium hexafluoroantimonate doesn’t mix well with water. That simple fact drives decisions across the supply chain, showing once again why digging deep into chemical properties serves everyone.
Diving Into the Chemical Structure
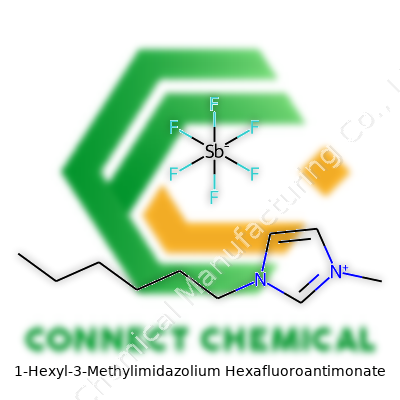
Digging beneath the long name, 1-Hexyl-3-methylimidazolium hexafluoroantimonate stands out as an ionic liquid—basically a salt that stays liquid at room temperature. The chemical structure features two main parts: the cation and the anion. The cation comes from the imidazolium family, which includes a five-membered ring with two nitrogen atoms and three carbon atoms. Tossing a methyl group onto the third position and a hexyl chain onto the first gives it the name “1-hexyl-3-methylimidazolium.” Its chemical formula: C10H19N2+.
The anion pairs up with the cation. In this case, it’s hexafluoroantimonate, carrying a chemical formula of SbF6-. This beast of an anion packs six fluorine atoms around a single antimony atom. When these two ions meet, you get the full salt: 1-Hexyl-3-methylimidazolium hexafluoroantimonate, with an overall formula of [C10H19N2][SbF6].
What Makes This Compound Significant?
Chemists love working with imidazolium-based ionic liquids because they rarely ignite and they don’t evaporate as quickly as many organic solvents. For anyone who’s ever splashed acetone on a bench top and watched half of it disappear in minutes, an ionic liquid feels almost magical. There’s also the benefit of low volatility—which matters a lot for safety in both industrial and research settings. Hexafluoroantimonate, the anion on the other side, lends even more chemical stability. It helps keep the liquid incredibly hard to break apart, so it stands up to tough chemical conditions.
My experience in the lab showed that using 1-hexyl-3-methylimidazolium hexafluoroantimonate can simplify tricky syntheses. It dissolves both organic and inorganic compounds pretty well, so reactions don’t grind to a halt while you stand there stirring away. That flexibility doesn’t just save time; it cuts down on waste, too. And anybody who’s sorted chemical waste drums knows how important that can be at the end of the week.
Safety and Environmental Considerations
Not everything smiles when it comes to this compound. Fluorinated anions, like SbF6-, raise eyebrows for environmental reasons. They do not break down quickly in nature and can end up persisting in water or soil. There’s an open conversation about the long-term impact of using hexafluoroantimonate salts or any ionic liquid with similar anions. Safe disposal often means handing things over to a chemical waste team instead of pouring the leftovers down the drain.
Seeking Smarter Solutions
Green chemistry doesn’t have to stay on paper. Researchers have begun pushing for ionic liquids that use less hazardous anions, swapping out antimony or fluorine for more environmentally friendly elements. Some teams use biodegradable anions sourced from natural products. In practice, substitution goes easier for certain applications than others. More funding for real-world testing could reveal new options that balance performance and safety, especially for large-scale industries.
Bottom Line
Knowing the structure and formula of 1-hexyl-3-methylimidazolium hexafluoroantimonate gives scientists a leg up on choosing solvents wisely. Choosing the right ones sets the stage for safer, cleaner, and more efficient chemistry. Innovation grows from this kind of understanding—grounded firmly in science, but always reaching for something better for both labs and the environment.