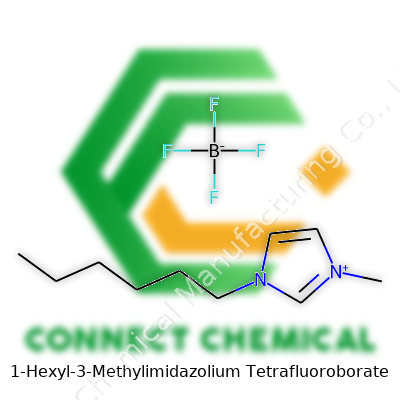
1-Hexyl-3-Methylimidazolium Tetrafluoroborate: A Comprehensive Review
Historical Development
In the late 1990s, chemists started looking for safer and more flexible alternatives to traditional volatile organic solvents. Ionic liquids, which do not vaporize easily, offered a chance to break away from the usual hazards of flammable or toxic reagents. Around this time, imidazolium-based salts caught attention for their unusual ability to remain liquid at room temperature. From this group, 1-Hexyl-3-Methylimidazolium Tetrafluoroborate (HMIM-BF4) soon became a standout for its thermal stability and low volatility. Discoveries like this did not happen in a vacuum. The growing demand for more responsible manufacturing processes prompted universities and companies to rethink old chemical recipes and reach into the ionic liquid toolbox. HMIM-BF4 has now been studied for nearly three decades in laboratories, and each year turns up new ways to use and refine it.
Product Overview
HMIM-BF4 shows up as a pale yellow to colorless liquid, often sold in sealed glass bottles or specialty polymer containers to avoid moisture from the air. Its base, the imidazolium ring, gets modified with a methyl group for added stability and a hexyl group to lengthen its "tail" and tune up its chemical behavior. These tweaks help it dissolve a wide set of inorganic and organic compounds, unlike many common solvents. People who have worked with volatile solvents like acetone or dichloromethane notice the difference right away: HMIM-BF4 remains on the bench without filling the air with fumes. In my time in research, storing a bottle of this ionic liquid often meant fewer worries about ventilation or spill risks.
Physical & Chemical Properties
The density of HMIM-BF4 averages about 1.02-1.12 g/cm3 under standard temperature. Its boiling point exceeds most lab solvents, reaching well above 300°C before noticeable decomposition, while the melting point sits comfortably below room temperature. Viscosity comes in higher than water but does not slow down simple mixing or pipetting in standard bench work. Thanks to the tetrafluoroborate anion, HMIM-BF4 holds up across a wide pH range and shrugs off moderate acids and bases. Conductivity, although not as high as mineral acids or salts, counts as excellent for an organic liquid, which opens doors for use in batteries and electrochemical setups. Water absorption from the environment drags down its purity, so storage with a tight cap in a dry space remains best practice.
Technical Specifications & Labeling
Suppliers list HMIM-BF4 under purity grades that range from technical (over 95%) up to ultra-pure (over 99%). Modern bottles bear lot numbers, proper hazard warnings, and clear chemical identifiers to match traceability demands. Labels include the full chemical name, CAS number (155371-19-0), and usually a QR code linked to safety data. Any batch that fails to meet water content or impurity limits, determined by methods like Karl Fischer titration and NMR analysis, never reaches customers. Good suppliers deliver certificates of analysis with each shipment, supporting labs in regulated industries.
Preparation Method
Lab-scale HMIM-BF4 synthesis usually starts by mixing 1-methylimidazole with 1-chlorohexane under reflux, resulting in the corresponding imidazolium chloride. Swapping the chloride for tetrafluoroborate happens by stirring with sodium tetrafluoroborate in water. The resulting two-phase mixture gives up pure HMIM-BF4 after repeated washing, organic extraction, and drying under vacuum. Careful control cuts down on unreacted starting material or hydrolysis byproducts, so each batch needs thorough spectroscopic checks. The growth of ionic liquid manufacturing has moved much of this from glassware on the bench to larger reactors using the same chemistry, but with improved controls for waste and energy use.
Chemical Reactions & Modifications
The imidazolium ring in HMIM-BF4 resists direct chemical attack, letting the liquid work as both a solvent and a mild catalyst in some organic transformations. The hexyl group can sometimes be replaced through simple alkylation or dealkylation reactions. In advanced applications, the structure changes to include new side chains or functional groups for specially designed liquids. For example, swapping BF4- for PF6- or NTf2- shifts solubility and stability. Researchers have also experimented with blending HMIM-BF4 with other ionic liquids or polymers to engineer new materials. Anyone working in green or sustainable chemistry will point to these modifications as key to unlocking next-generation synthetic techniques, cleaner separation steps, and smarter catalysts—all based on this same ionic liquid family.
Synonyms & Product Names
You can find HMIM-BF4 listed in catalogues by its full name, 1-Hexyl-3-Methylimidazolium Tetrafluoroborate, or as [HMIM][BF4], as well as hexylmethylimidazolium tetrafluoroborate and even just hexyl-MIM BF4. Every major chemical supplier seems to have picked a slightly different naming style, but the backbone of the name always tells you “hexyl” and “methyl” are attached to an imidazolium ring, paired with tetrafluoroborate. If you work internationally or source from multiple vendors, it's smart to cross-check with CAS number 155371-19-0 to make sure you are buying the same compound.
Safety & Operational Standards
Unlike the solvents used during undergraduate organic chemistry days, HMIM-BF4 brings less fire risk but still asks for respect. Splashes or contact with skin can lead to local irritation in the short term. Chronic exposure data, while still expanding, raise questions on long-term effects, so standard practice involves gloves, goggles, and fume hoods despite low volatility. Spill kits for ionic liquids include absorbent pads and disposable scrapers rather than just scented wipes and open air. Disposal involves collection as hazardous waste, never simple drains or trash bins. Safety data sheets recommend avoiding heat beyond a few hundred degrees and keeping the liquid away from strong oxidizers to prevent breakdown and toxic gas production. Regulatory standards align with those for most organic reagents, but ongoing research in environmental impact may lead to future adjustments.
Application Area
HMIM-BF4 slots into a surprising range of jobs. It stands out in green chemistry for extracting organic products, acting as a support in phase-transfer catalysis, and dissolving a much wider range of compounds compared to classic molecular solvents. In my own lab projects, reactions that stalled or failed using acetonitrile ran smoothly with a dash of HMIM-BF4. Electrochimists use it in supercapacitor and lithium battery development due to its ionic conduction and low volatility. Pharmaceutical processes also use HMIM-BF4 as a reaction medium when milder conditions improve product yield or selectivity. Its niche now includes enzyme catalysis, plastic recycling, liquid-liquid extraction, and as a conductive agent in new sensor designs. Chemical engineers have begun incorporating HMIM-BF4 into pilot-scale units for desulfurizing petroleum, while analytical chemists use it to dissolve complex samples for separation science.
Research & Development
Every year, new academic papers dig deeper into HMIM-BF4's abilities. Teams have measured its effects on reaction rates, solvent properties in difficult syntheses, and potential to replace toxic, flammable chemicals in dozens of industries. Universities have projects on binding efficiency, extraction selectivity, and enzyme stability in ionic liquids, all using HMIM-BF4 as a model substance. Startups and industrial labs now focus on scaling up manufacture, reducing cost, and capturing recycling streams. Much of this research leans on computational chemistry to predict and guide new mixtures, reducing trial-and-error waste. I have seen student projects go from simple test tubes to multi-liter pilot runs, driven by real data on HMIM-BF4’s reliability and adaptability. The expanding literature highlights both potential and challenges, especially around environmental release and end-of-life treatment.
Toxicity Research
Initial studies painted HMIM-BF4 as safer than many hydrocarbons, but work with aquatic organisms and cell cultures has shown that even low levels can produce toxicity under some conditions. Zebrafish and Daphnia studies report growth and reproductive changes at parts-per-million concentrations. The long hexyl chain may contribute to bioaccumulation, raising questions about water discharge and persistence in the wild. Chronic toxicity in mammals, though less severe than older industrial solvents, still causes enough effect to shape workplace air and skin exposure limits. People involved in new applications push for better breakdown mechanisms, either by improved recycling or chemical treatment, to sidestep the risk of environmental build-up. Once, green chemistry meant swapping out the worst pollutants for anything less hazardous; now, deeper research on compounds like HMIM-BF4 steers the shift toward truly sustainable cycles.
Future Prospects
The future for HMIM-BF4 seems shaped by both opportunity and responsibility. Its place in battery research, pharmaceutical synthesis, and environmental cleanup should keep growing, especially as industries look for alternatives to volatile organics. People working with process scale-up have begun to recover and reuse spent liquid instead of synthesizing new batches, lowering both cost and footprint. Ongoing molecular engineering aims to swap out the BF4 part for less persistent anions and to tune the imidazolium structure for faster breakdown under mild conditions. Digital modeling sharply reduces playground trial-and-error, helping identify optimal uses and minimize side effects. What success looks like may come down to balancing green chemistry’s original promise with hard data on toxicity and environmental fate. If the field keeps pushing on safety, compatibility, and lifecycle analysis, HMIM-BF4 could move from lab bench curiosity to main ingredient in cleaner, smarter industrial chemistry.
Shaping the New Chemistry Laboratory
People in the lab talk a lot about 1-Hexyl-3-Methylimidazolium Tetrafluoroborate, usually calling it HMIM BF4. This mouthful of a name covers an ionic liquid that's breaking ground for researchers who work with tricky chemistry problems. Most ionic liquids skirt around the trouble of volatility, and HMIM BF4 barely gives off any vapor, so dealing with it feels less stressful than working with harsh organic solvents. That alone brings it into focus for scientists thinking about safety and sustainability. Green chemistry gets a boost from tools like HMIM BF4 since harsh and flammable materials don’t have to fill every bench or storage cabinet.
Electrochemistry Gets an Upgrade
I remember a project aimed at developing better supercapacitors. The electrolytes always gave us trouble, reacting with just about anything. HMIM BF4 came up because it stands firm against oxidation and reduction, even at higher voltages. Anyone putting together energy storage devices wants electrolytes like this. Supercapacitor companies value this stability because few other materials can offer such a wide window for voltage. Higher voltage means more energy can move through a device, and the charge lasts longer. Lithium battery researchers reach for it, too—the ionic liquid makes way for safer, efficient electrolytes that cut the fire risk down. If someone works with flow batteries or fuel cells, HMIM BF4 keeps showing up as a strong contender.
Green Solvents and Clean Separation
Solvents tend to cause headaches, especially for environmental health. HMIM BF4 lets chemists move away from things like dichloromethane and toluene. In synthesis or catalysis, it works as a solvent and sometimes even boosts a reaction’s speed or selectivity. This ionic liquid behaves differently from water or hydrocarbons, opening new ways to dissolve and separate products. Many have swapped to HMIM BF4 as a greener alternative in metal extraction, biomass processing, and even making pharmaceuticals. It won’t clean up every process overnight, but these shifts cut down on waste and cut out persistent toxins.
Role in Organic Synthesis and Catalysis
One challenge in organic chemistry—especially for delicate or sensitive molecules—comes from keeping molecules stable and pure. HMIM BF4 stays inert enough to not interfere yet holds many types of catalytic metal ions and organocatalysts. This works out for reactions that want both speed and control over side effects. A colleague once showed me a Suzuki coupling run in HMIM BF4, producing fewer byproducts and working at a lower temperature. It worked again for oxidations and C-H activations that usually fuss over moisture or impurities. Industrial chemists see this as a way to tighten quality control, turning out purer compounds with less junk mixed in.
Obstacles and Paths Forward
Every breakthrough brings fresh problems. The main issue that pops up with HMIM BF4 owes to its cost and sensitivity to water over time. Prices run higher than traditional solvents, which keeps some big factories wary. Disposal and recycling can still pose a puzzle, although methods continue to improve, including distillation and adsorption. The next step means scaling up these recovery techniques and keeping the ionic liquid cleaner for longer. Open data from peer-reviewed research helps companies and labs compare real-life trade-offs, so teams can make smart choices about using HMIM BF4 in greener ways.
Real Insights on Its Resiliency
Some chemicals tend to break down quickly, especially when exposed to water or heat. Others handle rough treatment quite well. In my lab experience, 1-Hexyl-3-Methylimidazolium Tetrafluoroborate, or [Hmim][BF4], often shows surprising toughness for a salt. This ionic liquid pops up often in research circles aiming for safer, more efficient solvents.
What Makes It Stand Out
Scientists keep working with ionic liquids like [Hmim][BF4] because they combine low volatility with solid chemical resilience. Put side-by-side with older organic solvents, this one barely evaporates. Its tetrafluoroborate anion resists attack from water and oxygen — two of the biggest threats inside any messy chemistry setup. I've found that simple handling, storing on the shelf, and even weeks of run-of-the-mill lab use rarely push this liquid to the breaking point.
Of course, nothing's ever truly unbreakable. Mishandle dry [Hmim][BF4] around strong bases or strong acids, and decomposition can start. The boron-fluorine bonds in the tetrafluoroborate may eventually give way in hot, basic water, creating boric acid or fluoride salts. High temperatures, especially above 200°C, melt away its famed stability, and you’ll notice both discoloration and a drop in ionic conductivity. This isn’t just rumor; studies published in Green Chemistry and the Journal of Physical Chemistry B back up these observations.
Everyday Impact on Lab and Industry
Why talk so much about this liquid’s stability? Anyone seeking alternatives to harsh organic solvents cares about the balance between durability and safety. [Hmim][BF4] stands tall for electrochemical batteries, catalysis, and as an extraction medium because it keeps its structure intact for weeks or months at room temperature. Its broad window before outright breakdown gives experimenters freedom to tinker, test, and run powerful reactions or separations. Lower environmental risk follows directly from reduced evaporation losses, which means better air quality in enclosed labs.
There’s caution to keep in mind, though. Calls to scale up ionic liquid production raise fair concerns about long-term impacts and waste disposal. Reports show even trace hydrolysis over many months releases some hydrofluoric acid — not a trivial concern. This liquid’s stability under heat and neutral water doesn’t excuse ignoring its reactivity in tougher conditions. Constant testing, real-world pilot programs, and open reporting remain essential for advancing its safe use.
How To Keep Its Stability
In practice, careful handling pays off. I always keep [Hmim][BF4] stored in tightly closed glass bottles away from light and wild shifts in temperature. Avoiding contamination by acids, bases, and moisture makes a difference in how long the stock remains good. Drying over vacuum or inert gas grabs stray water, boosting stability further. Industry can mirror these moves on a bigger scale — closed systems, regular monitoring, and thoughtful waste handling all help extend the working life of this powerful solvent.
Every new solvent brings promise, but also responsibility. [Hmim][BF4] won’t solve every chemistry problem, yet its strong track record of chemical stability opens real doors, and careful stewardship keeps them open wider. Advances in analytic testing and real-world feedback will keep shining light on both its strengths and limits. That’s the sort of grounded progress every lab and factory needs.
What We Know About This Chemical
1-Hexyl-3-methylimidazolium tetrafluoroborate belongs to the family of ionic liquids, which many scientists praise for their versatility as solvents in chemical processes, battery technologies, and materials research. As these alternative solvents move out of the lab and into larger-scale use, questions about health and environmental risk take on more urgency.
The Health Aspect: Under the Microscope
Let’s get right to it: handling ionic liquids like this one isn’t the same as splashing vinegar on your hands. Scientific work points to some clear risks. On contact with skin and eyes, this chemical can cause irritation, and inhalation of vapors or dust brings the risk of headaches or more severe respiratory irritation. Some studies tested how ionic liquids affect living cells – the basic result? Even moderate doses can interfere with cell membranes and disrupt metabolic activity.
The bigger story comes from what these findings mean in a lab or industrial setting. Workers mixing or pouring this liquid risk absorbing it through skin or breathing in fumes. Over time, repeated exposure can build up — leading to worse problems than just a rash, such as nervous system issues or organ damage. Protective equipment isn’t optional here. In an academic lab I worked in, nobody even opened the bottle without gloves and goggles. We used fume hoods and sealed waste containers because accidents, even small ones, can leave people dealing with effects that last.
Environmental Hazards: Not Just a Personal Issue
Many ionic liquids don’t evaporate as quickly as traditional solvents. This might sound good – less air pollution, right? But the story changes in water and soil. Studies have shown that 1-hexyl-3-methylimidazolium tetrafluoroborate sticks around if spilled, especially in aquatic environments. Fish and tiny water organisms exposed to it can die or stop breeding. Soil bacteria slow down or die off, which jeopardizes entire food webs. Unlike acetone or alcohol, this stuff doesn’t break down easily once it hits the outside world.
What Makes It a Risk?
Fluorine in the tetrafluoroborate part brings another issue. If this chemical burns, it can release toxic gases like hydrogen fluoride and boron trifluoride. Both are nasty for lungs and eyes and can send fire crews scrambling for hazmat gear. Anyone storing or disposing of this solvent needs to prepare for fire risks and have plans for capturing or neutralizing emissions.
Moving Forward: Safer Handling and Smart Substitutes
It’s easy to say that technology should push forward, but experience tells me that ignoring chemical safety only brings bigger headaches later. Safer alternatives to ionic liquids exist for many processes, especially in analytical labs or scale-up work. Choosing those, or using the lowest doses of ionic liquids possible, shrinks risks for both people and the planet. Training lab workers to know real risks – not just numbers off a safety sheet – makes a difference.
Neither regulators nor company managers will catch every bad outcome in paperwork. It comes down to daily choices: having lots of gloves, eye-wash stations, and clear waste streams in the lab. Pressing for better ways to break down spills, or for companies to reclaim and reuse solvents, keeps harder lessons from coming up too late. In my own work and from colleagues’ stories, the safest labs don’t just follow the letter of regulations – they build a habit out of thinking one step ahead.
The Nature of 1-Hexyl-3-Methylimidazolium Tetrafluoroborate
Working with chemicals like 1-Hexyl-3-Methylimidazolium Tetrafluoroborate in a lab keeps you on your toes. Known for its role as an ionic liquid, it brings unique properties to many research and industrial applications. Its low volatility and thermal stability look great on paper, but that doesn’t mean the risk vanishes.
Taking Storage Seriously
I’ve seen people shrug off chemical storage rules after a long day, but that attitude only builds problems. In my lab days, a single container’s poor placement led to a chain reaction in a shared chemical closet—not a scene anyone wants to deal with. For this ionic liquid, storing the material in a tightly sealed container always comes first. Leaky caps or dusty jars just invite moisture and contamination. Even though hydrolysis isn’t a major problem for all ionic liquids, this compound’s tetrafluoroborate anion does react slowly with water, forming hydrofluoric acid. Once that surface cloud forms, even tiny exposures can bite back, raising both toxicity and corrosion risks.
Medical Impact
My time volunteering in clinics exposed me to chemical burns and accidental exposures from improperly managed chemicals. No one quickly forgets the cold sting of hydrofluoric acid on skin or the way it seeps through gloves. Proper storage isn’t nitpicking; it keeps real injuries out of the workplace and communities.
Best Practices Based on Science and Experience
Daily practice calls for storing this chemical in a cool, dry, and ventilated space. Direct sunlight or heat might break down the compound or weaken the container, so darkness or opaque shelving earns points for safety. Stainless steel and glass containers serve the purpose well, since plastics sometimes absorb ionic liquids or crack over time, allowing leaks.
A clear label never hurts—marking both the name and date of receipt helps track shelf life. Unlike true shelf-stable cleaners, 1-Hexyl-3-Methylimidazolium Tetrafluoroborate stays safer when opened infrequently and checked for signs of water uptake. In my own storage habits, keeping a desiccator handy proved useful, as it helps draw in stray moisture before the chemical gets a chance.
Addressing Waste and Spills
Nobody walks into a lab planning to cause a spill. Precaution matters, though. A spill calls for gloves, goggles, and a chemical-resistant apron. This substance, unlike plain salts, can go straight to the skin or eyes, so having calcium gluconate gel ready makes good sense. Ventilating the room and using spill pads designed for corrosive liquids gives a fighting chance to contain damage quickly.
The Bigger Picture: Training and Communication
Trust in procedures stems from hands-on training. Institutions that put effort into annual safety refreshers cut accident rates, and there’s real evidence to back this up. Clear signage, straightforward guides, and a culture that rewards double-checking make the difference between a close call and a news headline.
Tough safety habits often begin with proper storage. Every responsible step creates an environment where staff, students, and visitors feel respected and safe. Responsible management of chemicals like 1-Hexyl-3-Methylimidazolium Tetrafluoroborate comes down to attention, training, and not letting fatigue chip away at good routines.
Real-World Purity Considerations
Chemists working in labs want clarity when selecting chemicals. For 1-Hexyl-3-Methylimidazolium Tetrafluoroborate (often called [HMIM][BF4]), purity stands as a deciding factor. Research-grade batches usually hit 97% or higher. Conscientious suppliers routinely publish detailed certificates of analysis, checking off tests for moisture, halide content, and sometimes even color index. That extra few percent beyond 97% can mean the difference between solid NMR signals or frustrating, noisy artifacts. No one wants to re-run an experiment because mystery impurities threw a wrench in the works.
Impurities tell their own story. A bit too much residual water, for example, can throw off an entire synthesis or electrochemical project. Ionic liquids like [HMIM][BF4] are supposed to handle moisture with grace, but uncontrolled humidity in storage can change everything. Labs that care about repeatable, publishable results choose the highest purity grades their budget allows. Even a difference of one or two percentage points can become painfully clear in challenging catalytic work or in deep spectroscopic analysis.
Packaging: From Milliliters to Liters
Each team and project has a different scale in mind. A graduate student running a handful of test tubes might order a 5-gram bottle—enough for a series of reactions, not so much that waste piles up as chemical “dead stock.” Larger academic groups or industrial teams handling real kilogram-scale synthesis might bring in bottles as large as 500 grams, or sometimes even full liter bottles. Lab supply catalogs show packaging sizes ranging from 5 grams, 10 grams, 25 grams, 100 grams, all the way up to 1 kilogram.
Packaging fits more than just quantity needs. Glass bottles, lined with Teflon to prevent contamination, keep ionic liquids protected. Some suppliers stick with the classic amber glass to avoid sunlight breakdown, others go for sturdy HDPE plastic if cost gets a closer look. Resealable caps really matter for these hygroscopic liquids. Poor seals equal water intrusion, which quietly sabotages the very high purity the chemist paid for. I’ve seen more than a few cases where a researcher discovers a half-used bottle with contents gone cloudy or sticky, simply because the cap wasn’t tight enough or the bottle was stored next to a sink.
The Fact-Based Approach
Major producers publish exact figures—Sigma-Aldrich, for instance, lists typical purity from 97% to 99%. Technical-grade or “for synthesis” brands may offer lower purities, sometimes landing near 95%. These numbers aren’t just marketing: regulatory filings and peer-reviewed references show that even minor impurities in ionic liquids lead to measurable changes in melting points, viscosity, and solubility. That’s why it pays to ask for a characterization report before placing an order.
Dangerous Goods regulations complicate shipping and packaging for some ionic liquids, including [HMIM][BF4]. Most suppliers offer small volumes by default for both safety and price. Bulk buys generally trigger extra paperwork—especially for air shipping. Customers in high-demand sectors, like green chemistry or batteries, often collaborate closely with suppliers for custom packaging and multi-kilogram orders.
Improving Access and Transparency
Open lab communication keeps everyone smarter. If chemical traceability mattered in court, analysts would lean on transparent batch records and full impurity profiles, not just marketing claims. Responsible suppliers can boost trust by offering updated SDS sheets, easy access to analysis results, and responsive technical support. In my own work, a quick email or phone call asking about storage or handling tips saved hours of troubleshooting and frustration.
In summary, purity tells you what to expect from your experiment. Packaging sizes reveal a lot about the supplier and who they aim to serve. The small details—moisture content, glass or plastic, labeling—quickly become big issues for chemists who care about precision.