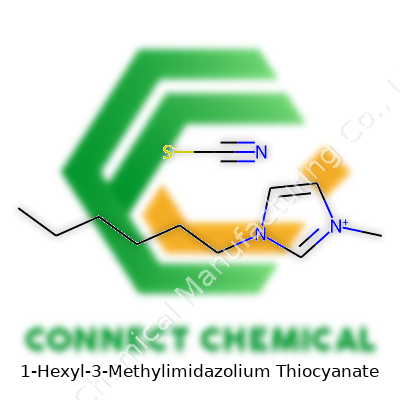
1-Hexyl-3-Methylimidazolium Thiocyanate: Insightful Commentary
Historical Development
Decades ago, most chemists focused on traditional organic solvents, often ignoring their downsides in favor of cost and routine. Imidazolium-based ionic liquids changed that tune, offering new approaches for dissolving stubborn compounds or tailoring special conditions in reactions. When scientists first tinkered with 1-Hexyl-3-methylimidazolium thiocyanate, the goal wasn’t glamour or a race to market—it was necessity. Conventional solvents brought fire hazards and pollution, so the push for safer and reusable alternatives grew. The most compelling breakthroughs didn’t come from massive companies, but from small research labs that made steady progress—step by step. These efforts laid the groundwork for green chemistry initiatives, and the collective push toward ionic liquids like this one owes a lot to those who rolled up their sleeves and asked, “Can we do this better?”
Product Overview
1-Hexyl-3-methylimidazolium thiocyanate stands out among ionic liquids for its distinct combination: a long alkyl chain coupled with a versatile imidazolium core and the reactive thiocyanate anion. This structure impacts its interaction with both inorganic and organic molecules. In the lab, you’ll find it as a thick, often colorless liquid, not the powder or crystalline stuff that gets everywhere. That makes it less messy and easier to handle. It doesn’t evaporate away or catch fire under normal conditions, which matters a lot to anyone working on a cramped lab bench breathing fumes day in, day out.
Physical & Chemical Properties
Its melting point hovers below room temperature, so it stays liquid even in cool labs. Viscosity remains high and noticeable—pour it out and you don’t see it running free; it crawls. It dissolves many salts and organic chemicals thanks to that long nonpolar chain and charged ring. Water solubility varies, but the organic side opens doors for dissolving greasy or hydrophobic materials—something I stumbled upon during my time working with stubborn organic extractions. It does not give off sharp odors, and unlike so many solvents, it refuses to ignite at standard temperatures, making it safer to store and use even when someone forgets to check the thermometer or fume hood.
Technical Specifications & Labeling
Any bottle on the shelf comes with a clear label: chemical name, formula (C10H19N3S), purity usually listed above 98%. The material safety data sheet will mark hazard categories—irritant or low-toxicity, not as dire as older halogenated solvents. Density sits near 1.0 g/cm³, and thermal stability stretches past 200°C, keeping degradation off the checklist for most bench reactions. The storage advice comes down to dry, dark, and sealed—common sense for anyone who’s watched a reagent bottle crust over after a month exposed.
Preparation Method
Preparation starts with methylimidazole and hexyl halide, reacting these at moderate temperatures followed by the addition of potassium or sodium thiocyanate in a two-phase system. The organic phase gets washed and dried. Direct scale-up works, but controlling moisture and using glassware with ground joints really pays off. If you’ve done basic organic synthesis, this workflow feels familiar—mix, separate, purify, repeat. Careful folks might throw in an extra purification step using activated charcoal to polish the final product. Crude versions sometimes carry halide traces or off-odors that a quick water wash or slow vacuum drying can handle.
Chemical Reactions & Modifications
This ionic liquid doesn’t just sit there; it shapes reactions. Whether it’s acting as a solvent in nucleophilic substitutions or stabilizing reactive intermediates, its unique ion pairing helps. With heat or careful catalysis, it can exchange its thiocyanate for other soft nucleophiles or form complexes with transition metals, which sometimes helps build catalysts. Swapping the alkyl group or ring modifications lets chemists customize how it dissolves specific molecules or interacts with different metals. Anyone trying to push green synthesis has at least considered it, thanks to its adaptability and ability to speed up or select for new pathways. From dissolving cellulose to creating new catalytic systems, real-life reactions keep scientists thinking up new uses.
Synonyms & Product Names
You might see it labeled as [HMIM]SCN, 1-hexyl-3-methylimidazolium thiocyanate, or even hexylmethylimidazolium thiocyanate. Some catalogs abbreviate it as C6mimSCN or list it under specific registry numbers. Cross-checking between suppliers avoids mix-ups—especially when ordering for a critical experiment or pilot batch. One missed letter in the abbreviation can bring in the wrong variant, wrecking controls and wasting precious time.
Safety & Operational Standards
Long shifts in the lab have shown that no chemical truly feels “safe,” but this one ranks lower on the danger scale. Common sense PPE—nitrile gloves, goggles, lab coat—does the trick, and a working fume hood makes for peace of mind. Spills don’t vaporize into toxic clouds. Local legislation, especially in Europe and North America, tracks ionic liquids for environmental impact even though the direct risk stays low. Waste management systems flag it for organic waste, keeping it out of waterways. Having handled dozens of solvents while training new scientists, I’ve seen that risk drops when people understand the why behind these standards—not just the checkboxes.
Application Area
Its main playground is the lab, but innovation leaks out into pilot production lines. Chemists use it to replace volatile organics in reactions ranging from halide substitutions to alkylations. Electrochemists measure its window for battery and capacitor research. There’s interest in using it to dissolve cellulose for specialty polymers, or capturing CO2 as industries try to bootstrap their process sustainability. Analytical chemists appreciate its non-volatile nature for extractions and chromatography, especially when standard solvents pull down air quality in small labs. Scale-up for industrial use still lags because of price and regulatory inertia, not because of the molecule’s performance.
Research & Development
Current research goes after greener synthesis routes and recyclability. R&D labs focus on tweaking side chains to dial in melting point and hydrophobicity, hoping to match specific tech demands like better electrolyte systems for batteries or unique dissolution profiles for biomass refining. Comparative studies on its solvent power, thermal stability, and electrochemical properties fill out journals and conferences. Government and university projects pile up data on lifecycle analysis, eager to prove that these liquids beat classic solvents not just in function but across the entire chemical lifespan—from cradle to grave.
Toxicity Research
Long-term safety studies dig into environmental persistence and bioaccumulation. Initial data shows limited acute toxicity. Direct skin exposure brings only mild irritation for most people, though no one should treat it lightly. Fish and aquatic organisms provide tougher checkpoints; their sensitivity means ongoing studies keep a close eye on emissions and accidental releases. Some early bioassays suggest degradation products could build up over time, so disposal rules put tight controls for rinsate and spent stocks. My review of recent journal articles confirms that research councils want full toxicological profiles before these liquids take over legacy solvent slots in industry.
Future Prospects
Seeing where chemistry heads next, these ionic liquids will anchor new technologies for green engineering. The real test comes from industry clamoring for safer, recyclable solvents that keep energy use and waste low. Chemists look to blend them for custom functions: dual-role solvents, catalysts, and stabilizers—often in a single molecule. Cost pressures will drive innovation in synthesis and recycling methods. Education and clear industry guidance will steer adoption, but the demand for better solvents has always come from those who care about both the bottom line and the planet we leave behind. Long days at the bench show me that change comes slow, but persistence and the right tools eventually shift the whole field forward.
Getting to Know 1-Hexyl-3-Methylimidazolium Thiocyanate
Science moves forward because people ask questions, break down tough molecules, and get their hands dirty in labs. Whether working at the university or inside an industry lab, the work often starts with understanding a simple thing: the chemical formula. With 1-Hexyl-3-Methylimidazolium Thiocyanate, known for its place in ionic liquid chemistry, this step steers the whole research direction.
Breaking Down the Structure
This compound falls in the family of ionic liquids. Folks working in battery tech, organic synthesis, or green chemistry care about these because they’re liquid at room temperature, don’t evaporate like old-school solvents, and often help reactions that otherwise stall. The formula for the cation, 1-Hexyl-3-Methylimidazolium, gets written as C10H19N2+. It’s got a hexyl chain hanging off the imidazolium ring (which is just two nitrogen atoms stuck inside a five-membered carbon ring), plus a methyl group giving it some bulk.
Thiocyanate, the anion partner here, sits at the other end as SCN-. This little group shows up in all kinds of places—fire retardants, photographic chemicals, and lab reagents. You slap these two pieces together, and the result is a charged pair: C10H19N2+ SCN-.
Why the Formula Matters in the Real World
Take work in sustainable solvent development. If you can swap a toxic or flammable solvent with an ionic liquid like this one, less pollution and lower safety risk can follow. The precise makeup—those ten carbons, nineteen hydrogens, two nitrogens—shapes everything from melting point to how it interacts with metal ions. Battery research, for instance, benefits because these ionic liquids can up the energy density or lifetime of a cell. SCN-, as an anion, lends character that can help dissolve metal salts, clean up catalyst leftovers, or let researchers fine-tune the ionic conductivity.
Some labs search out even more exotic combinations, but the building blocks stay close to basics. You start with the chemical formula. Every shelf in an active research lab has bottles labeled with formulas. Safety protocols require knowing exactly what sits in each bottle, since properties—from toxicity to flash point—depend on those building blocks. Someone handling a bottle marked with C10H19N2SCN doesn’t just see a jumble of letters; they see years of development, a tool for prototyping, or a risk factor if mishandled.
Addressing the Issues and Moving Forward
Not every lab can buy up dozens of ionic liquids. Supply chains, production costs, and regulatory clearances restrict access, especially in places with fewer research dollars. Students working on tight budgets might mix these compounds from scratch, with risks if they don’t pay close attention to lab protocols. Labs need up-to-date Material Safety Data Sheets and routine staff training. Sharing best practices through research groups and open-access sites helps more people come in contact with safer, smarter chemistry.
Opportunities keep coming for more sustainable chemistry, especially if recycling and reuse programs for ionic liquids grow. Some folks are already reclaiming spent solvents instead of dumping them, shrinking waste and operating costs. Better knowledge of each compound’s formula, properties, and lifecycle—from lab to landfill—keeps this movement grounded in real science instead of hype.
Summary
The chemical formula for 1-Hexyl-3-Methylimidazolium Thiocyanate is C10H19N2SCN. Behind these letters is a whole world of lab work, safety checklists, and creative problem-solving—a reminder that good science starts with knowing what's inside the bottle.
Everyday Chemistry Gets a Modern Twist
Some chemicals seem abstract until you see the hands-on work they do. Take 1-Hexyl-3-Methylimidazolium Thiocyanate—let’s call it HMIM-SCN for clarity. This ionic liquid stands out not just for its unpronounceable name but for the jobs it handles in the real world. Unlike water or oil, it doesn’t evaporate or combust fast, and that changes the game in labs and on the factory floor.
Helping Researchers Separate and Clean
People in chemical separation run into barriers. Many solvents can’t dissolve tough salts, and good luck dealing with stubborn organic compounds using just the classics. HMIM-SCN pushes those walls aside. Its ability to mix with polar and non-polar substances helps chemists drag out what they need without juggling multiple chemicals. I spent hours in school labs fighting with solvents that stunk up the place and struggled with efficiency. With ionic liquids like this one, extractions get faster and often safer. The low vapor pressure means a reduced risk of fumes—less headache, both literally and figuratively.
Electrochemistry Runs Smoother
Batteries and supercapacitors don’t just depend on metal and plastic. The electrolytes running the inside need to hold and transport ions under tough conditions. HMIM-SCN can do that at room temperature, without drying out or catching fire. Having worked near industrial battery research, I learned that swapping in safer, more stable liquids can prevent entire testing runs from landing in the scrap bin. The performance bump from a steady ionic environment means less downtime and equipment loss.
Catalysis Gets a Lift
Catalytic reactions usually crave high yields and speed. Traditional solvents sometimes slow things down or kill selectivity. Swapping in HMIM-SCN sometimes leads to faster turnarounds because it dissolves both metal catalysts and organic substrates better. In pharma labs, where time is money, I’ve seen researchers shave hours off syntheses with ionic liquids. The fewer byproducts you get, the easier the cleanup—no one enjoys tedious purification steps after a long shift.
Green Chemistry Opportunities
Regulatory pressures push everyone toward greener processes. HMIM-SCN carries low volatility, so accidental releases mean less environmental impact. Solvents that don’t quickly evaporate reduce the risks for workers too. I remember hearing from an industrial chemist who switched to ionic liquids to dodge fire hazard complaints and avoid daily personal protective gear suited for organic solvent spills. The economic costs tied to workplace injury drop, and so does regulatory red tape.
The Roadblocks and a Broader Solution
No chemical gets a free pass. Despite its perks, HMIM-SCN sometimes brings disposal headaches. Ionic liquids don’t always break down easily, so waste management matters. The cost runs higher than everyday solvents, too. I see a future in recycling programs where facilities reclaim and regenerate these liquids instead of treating them as one-shot solutions. Some companies already install small-scale purification setups on-site, cutting disposal bills and earning points with sustainability auditors.
The Bottom Line
Chemists, engineers, and industry workers see ionic liquids as more than a trend. Experience and results show their value in extraction, electrochemistry, and catalysis. HMIM-SCN’s mix of stability and function gives people across chemistry the tools to solve problems old solvents couldn’t touch, so long as we stay sharp about safe use and smart disposal.
Understanding the Chemical
1-Hexyl-3-methylimidazolium thiocyanate belongs to a class known as ionic liquids. Chemists and engineers love these liquids for their unique abilities: non-volatility, thermal stability, and a knack for dissolving all sorts of things. In labs, researchers put them to work in green chemistry, battery research, and specialized extraction, chasing breakthroughs that could change how things get made.
Digging Into the Hazards
The safety story of 1-hexyl-3-methylimidazolium thiocyanate isn’t clear-cut. Unlike widely used solvents like acetone or methanol, this ionic liquid doesn’t pop up much in basic toxicology manuals. Its makeup should make people pause: the imidazolium ring brings some reactivity, and coupled with the thiocyanate anion, you’re looking at a compound that rolls out of the lab with some red flags attached. I’ve watched careful chemists handle similar ionic liquids—thick gloves, full eye protection, lab fume hood always humming.
Research points to risks. Some imidazolium-based ionic liquids show toxicity toward aquatic life. A 2020 report published in Chemosphere flagged several of these liquids as harmful to freshwater algae and fish. Acute and chronic effects sometimes pop up even at low concentrations. Scientists don’t always focus on 1-hexyl-3-methylimidazolium thiocyanate in isolation—but with the same molecular framework, safety profiles often overlap.
On top of that, thiocyanate by itself raises concerns. It can mess with thyroid function in people and animals after chronic exposure. Industrial chemists know to keep an eye on bioaccumulation, too. One slip during disposal, and there’s a risk: improper waste handling puts waterways and local wildlife at risk, even if current production volumes stay low.
Personal Experience in the Lab
In my own work with ionic liquids, strict routines keep exposure as close to zero as possible. Full-length nitrile gloves, goggles with good side coverage, and a reliable fume hood form the first layer of defense. I schedule extra time for safe transfers and clean any spills with plenty of adsorbent—never paper towels or regular sinks. Mistakes cost time and, in worst cases, health.
I’ve seen overconfident juniors learn the hard way that “green” doesn’t mean safe. Ionic liquids don’t waft through the air like chloroform, making them feel safer, but they can linger on skin and materials where they don’t belong. Even a little on the bench can cause skin rashes or trouble for anyone cleaning up without the right gear. That caution gets baked into our protocols for good reason.
Weighing the Importance of Safety Data
Anyone interested in this chemical should look up its Safety Data Sheet (SDS). Large suppliers make these public, and they tell the real story: keep away from skin, avoid breathing dust, store with good ventilation, and prevent releases into the environment. Until better long-term studies show otherwise, skepticism stays warranted. Regulatory agencies in Europe and the US both move slowly on new ionic liquids, and sometimes that lag leaves real-world risks underexplored.
Possible Steps Forward
Lab managers, educators, and professionals should drill safety from day one. Substitute less hazardous materials if possible. Use personal protective equipment, contain spills immediately, and never dump unused liquid down the drain. Disposal through hazardous waste channels isn’t just best practice, it’s the only responsible route. At the same time, funding bodies and policymakers should prioritize full-scale toxicity studies for this whole class of chemicals, not just a handful. Until that data lands, the most sensible move is caution linked closely to respect for the unknown.
Why Storage Matters for Chemical Safety
Seeing a label like 1-Hexyl-3-Methylimidazolium Thiocyanate sparks a few thoughts in anyone who has spent much time around labs or chemical warehouses. Tinkering with new materials, I always took extra care with ionic liquids like this one. Mishandling leads to expensive mistakes, ruined research, or even health hazards. The right storage steps matter as much as knowing the difference between water and ethanol during a titration.
Understand the Properties Before Setting It on a Shelf
1-Hexyl-3-Methylimidazolium Thiocyanate comes from the same family as other imidazolium-based ionic liquids. It usually appears as a colorless to light yellow liquid and comes with the perks of ionic liquids: low volatility, decent chemical stability, but still a sensitivity to moisture and air in many cases. It reacts with strong oxidizers and is hygroscopic, meaning it draws water out of the air, and that can change its properties. Pooled water inside a bottle may seem harmless, but the change can affect everything from reaction performance to safety.
Practical Storage Steps Backed by Experience
Keep it in an airtight container, without excuses. I always check for glass bottles with screw tops or certified-seal plastic, as this keeps both air and humidity out. A desiccator cabinet, or at least a sealed storage bin with silica gel packets, gives another line of defense against moisture. If your lab carries those reusable desiccant canisters, use them—it pays off in time and trouble prevented.
Store it out of direct sunlight to prevent unwanted reactions or degradation. I still remember a bottle left in a sunny window for a week, which led to color change and unusable material. My colleagues sorted the mess with a long sign-out form and some unkind words for the original user. Choose a cool, dry spot. Fluctuating temperatures speed up chemical breakdown, so avoid areas near heating vents, radiators, or busy windowsills.
Label everything clearly with contents, hazards, and the date the bottle was opened. Running inventories every few months helps as old materials can become unpredictable. Don't overlook secondary containment. Use a tray or bin to contain leaks, which catches spills before they spread across shelves or floors.
Supporting Safety with Authoritative Sources
Material Safety Data Sheets (MSDS) published by major suppliers back up these handling and storage habits. Sigma-Aldrich, Alfa Aesar, and Fisher Scientific provide guidelines that reinforce what works: keep it cool, dry, tightly sealed, and away from incompatible chemicals like oxidizers or acids. Check the MSDS or safety card every time you set up a new storage area.
Committing to these steps cuts down on errors, protects health, and saves budget in the long run. I’ve seen research halted by careless storage decisions, but I've also seen smooth work thanks to consistent practices. Good habits and clear communication about storage turn a bottle of 1-Hexyl-3-Methylimidazolium Thiocyanate from a risky mess into a reliable resource.
Purity Options on the Market
Walk into any specialty chemicals catalog and you’ll spot 1-Hexyl-3-Methylimidazolium Thiocyanate, often tagged as an ionic liquid popular with researchers and those who work in electrochemistry or material science. Purity levels usually hover around the 95% mark or higher, with some suppliers listing purities up to 99%. A common figure you’ll see is 98%, as that strikes a balance between cost and reliable lab results.
Why Purity Level Matters
Every time a new experiment fails to deliver consistent results, the first thing I’ve learned to examine is the substance itself. An impurity content, even as little as two percent, has been enough to sway data in battery electrolyte work and dye-sensitized solar cell projects. Ionic liquids like this can be sensitive. A stray contaminant, maybe leftover starting material or moisture, can mess up conductivity, color, and even stability. If a researcher tries to push boundaries in green chemistry or seeks perfect repeatability, every decimal of purity starts to count. Published data supports this—one study found that reducing impurities led to up to 15% greater efficiency in photovoltaic applications using this salt.
How Purity Is Tested
Most reputable chemical distributors post certificates of analysis that spill the details: moisture level, halide content, and absence of volatile organics. On my bench, running NMR or ion chromatography would give confidence. Some purists prefer suppliers who run Karl Fischer titrations for water, since moisture has a knack for sneaking in, especially if the bottle’s been on the shelf too long or sees air too often. A clean chromatogram or NMR spectrum builds the case for paying that extra premium for a higher purity grade.
What Influences the Choice
Deciding on a purity level isn't just about budget. Research rules shape the decision, too—journals or peer reviewers often demand high purity reagents. My old professor used to repeat, "You can't trust your numbers if you don’t trust your chemicals.” That's a tough lesson if you’re on a grant deadline or need to hit certain environmental standards. On the flip side, in some industrial trials or pilot-scale runs, there’s room for compromise, because a small impurity may not impact bulk processes in the same way.
Cost and Sourcing Issues
Higher purity always drives the price higher, especially as chemical suppliers invest in extra purification steps. In my experience, the step from 95% to 99% often doubles the price, but not everyone needs the high end. Sourcing from trusted suppliers helps reduce the chance of surprise impurities. Sometimes local supply chains have limited options, so global suppliers like Sigma-Aldrich or Merck become standard stops. Since most labs can’t spend weeks cleaning up chemicals, paying for the analysis and assurance often brings peace of mind.
Moving Toward Reliable Results
Quality control needs more than just a number on a bottle. I always recommend colleagues check batch-to-batch consistency and save a small amount from every purchase for reference. Reporting the exact purity in publications or industry documentation creates transparency and gives future users confidence in the process. Pushing suppliers to keep certificates detailed and updated helps everyone down the line.
Looking Ahead
People want cleaner, more reproducible science, and ionic liquids like 1-Hexyl-3-Methylimidazolium Thiocyanate sit right at the center of this demand. With better industry standards, accessible equipment for checking purity, and strong habits in documentation, the world gets more reliable results—and that benefits everyone from students in the lab to manufacturers bringing new technologies to life.