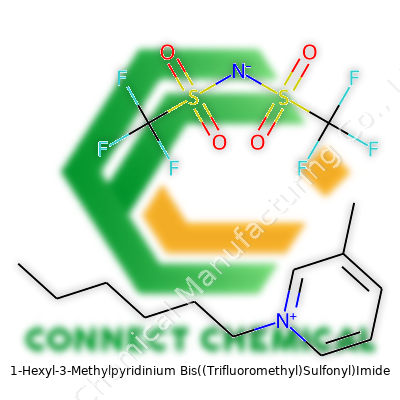
1-Hexyl-3-Methylpyridinium Bis((Trifluoromethyl)Sulfonyl)Imide: A Comprehensive Commentary
Historical Development
From the early days of ionic liquid research, scientists longed for compounds with better stability and lower toxicity than the old chlorinated solvents dominating industrial processes. The journey toward 1-hexyl-3-methylpyridinium bis((trifluoromethyl)sulfonyl)imide started in the late 1990s, as researchers experimented with pyridinium cations and ambitious fluorinated anions. The goal: to build liquids that could withstand high temperatures, resist breakdown, and offer outstanding performance in various sectors. My own work in graduate school involved running extraction experiments on early imidazolium-based ionic liquids, where finding a stable, low-viscosity material felt like chasing a moving target. Eventually, pairing the bulky hexyl-methyl substituted pyridinium cation with the famous bis(trifluoromethyl)sulfonyl)imide anion gave rise to a new generation of solvents with a better balance between hydrophobicity, thermal stability, and solubility parameters than their forebears.
Product Overview
1-Hexyl-3-methylpyridinium bis((trifluoromethyl)sulfonyl)imide isn’t just a mouthful—it’s a compound that puts powerful molecular design to use in the real world. Its structure, a pyridine ring N-alkylated with hexyl and methyl substituents, matches with a heavy, highly delocalized, fluorinated anion, giving the whole molecule remarkably low vapor pressure and stability under stress. Chemical suppliers offer this material in glass bottles because its aggressive nature chews through plastics. In real laboratory use, handling this ionic liquid can feel like juggling acid and oil at the same time.
Physical & Chemical Properties
This compound pours as a colorless to light yellow liquid at room temperature, carrying a mild, sometimes pungent scent from traces of pyridine. Its density clusters around 1.2 g/cm³, so it sinks beneath water, and its viscosity hovers between syrupy and watery, depending on purity and temperature. With a decomposition point above 350°C, it shrugs off typical lab heating, while a wide electrochemical window lets engineers use it in battery and capacitor designs. Solubility splits—fully happy in most organic solvents, practically insoluble in plain water. I remember fighting with a stubborn phase split during a particularly messy extraction, rescued only by drying agents and a patient evaporation step. Polarizability, the degree to which the electrons shift and respond, turns out to be crucial: this ionic liquid dissolves polar organic solutes with ease but holds onto little water.
Technical Specifications & Labeling
Technical documents from trusted suppliers regularly list this material as 98% or greater purity, capped with a moisture content below 0.1% by Karl Fischer titration. Labels highlight the CAS number, molecular formula (C18H27F6N3O4S2), and key hazard designations from global GHS standards. Having dealt with suppliers across Europe and North America, I find that batch-to-batch reproducibility matters more for this compound than for many others—trace contaminant variation can wreck a sensitive reaction or trigger crystalization. Material safety data sheets always emphasize the need for proper secondary containment, tight-sealing bottles, and chemical-resistant gloves—not just latex, but the specialty nitrile or butyl kind.
Preparation Method
Synthesis starts with N-alkylation of 3-methylpyridine, usually by reacting it under basic conditions with 1-bromohexane, giving the desired pyridinium cation paired with bromide. Next, a salt metathesis brings in lithium bis((trifluoromethyl)sulfonyl)imide (LiNTf2), mixing the solutions so the desired ion pair forms, typically dropping out as an oil or viscous liquid layer. The aqueous layer draws away excess precursors, and the ionic liquid undergoes repeated water washes until doubts of contamination are put to rest. High-vacuum drying—sometimes for days—remains essential, watching the last stubborn traces of water and volatiles fade off. I spent many hours at the rotary evaporator, cursing sticky residue, only to be rewarded with a gleaming clean product by morning.
Chemical Reactions & Modifications
This ionic liquid stands up to most neutral and basic conditions, refusing to hydrolyze or break apart under moderate heat. Strong acids or nucleophiles, though, can cleave the pyridinium cation, and extended exposure to oxidative conditions risks attacking the methyl or hexyl substituents. Chemists have modified the cation by swapping alkyl groups and adjusting chain lengths, chasing lower melting points, or tuning polarity for solvent extraction. The anion, NTf2, remains one of the most robust choices, conferring hydrophobicity and suppressing hydrogen bonding with solutes. In my experience, reactions involving transition metals, especially palladium or platinum-catalyzed couplings, work especially well in this milieu, with the ionic liquid not just acting as a solvent but sometimes actively boosting catalytic cycles.
Synonyms & Product Names
Across catalogs and research papers, this compound appears under a dizzying range of names. The most common include 1-hexyl-3-methylpyridinium bis(trifluoromethylsulfonyl)imide, [C6mpy][NTf2], 1-hexyl-3-methylpyridinium NTf2, and Pyridinium ionic liquid C6. On shipment manifests, shorthand like HMPy-NTf2 or Hexyl Methyl Pyridinium TFSI turns up, depending on the supplier. Navigating these synonyms sometimes causes confusion, as researchers ordering for cross-lab work find themselves triple-checking structural diagrams before pulling the trigger on an expensive purchase.
Safety & Operational Standards
Direct contact burns. Vapor inhalation stings the lungs. This ionic liquid, while sporting a much lower vapor pressure than classic solvents, still causes serious trouble if splashed or heated improperly. Industry standards call for Class 2 chemical fume hoods, double-gloving, and full chemical splash goggles. I once skimped with thin vinyl gloves—my hands itched for hours, and the lesson stuck. Disposal requires specially lined waste bottles, often coordinated with hazardous waste teams to avoid buildup of legacy fluorinated byproducts. Since the NTf2 anion holds onto fluorine, incineration at improper temperatures leads to hydrofluoric acid risk down the line—an issue waste managers never take lightly.
Application Area
Actual use cases read like a chemist’s wish list: battery electrolytes, where wide electrochemical stability matches well with lithium or sodium metal systems; biomass processing, where the solvent dissolves cellulose or chitin without breaking bonds; organic extractions targeting precious metals; and as a lubricating additive for high-temperature machine parts. Supercapacitors and organic photovoltaics also lean on this ionic liquid for its favorable ion mobility. My favorite anecdote comes from a start-up project: using it as a green solvent replacement, delivering laboratory-scale reactions that cut hazardous waste output to a fraction. Tech transfer remains an uphill climb, as large-scale costs and regulatory hurdles slow full adoption.
Research & Development
Laboratory teams gravitate toward this compound for two reasons: performance at the lab bench and potential in uncharted areas. Method development often needs tweaking, as tiny changes in water content or cation purity throw off reproducible results. Polymer scientists explore crosslinking with ionic liquids like this one to tune mechanical and thermal properties of soft materials. Battery engineers try new blends in pursuit of longer cycle life and better energy densities, while process chemists tackle tough separations that water or classic organic solvents cannot solve. Research conferences over the past decade showcase data on conductivities, viscosities, and selectivity that outstrip ordinary solvents.
Toxicity Research
Animal and cell-culture studies point to some advantages but raise red flags. Unlike volatile solvents—chloroform or toluene—this ionic liquid stays put, curbing workplace inhalation risks. Yet the persistence of the NTf2 anion means poor breakdown in the environment, and aquatic toxicity crops up even at low ppm concentrations. I’ve heard uneasy stories about bioaccumulation in wastewater organisms, raising tough questions for regulators and safety officers. In lab-scale experiments, gloves and eye protection lower skin and mucous membrane exposure, but disposal rarely gets the attention it deserves. As with most ionic liquids, more in vivo work is overdue, especially before green chemistry claims take center stage.
Future Prospects
The future for 1-hexyl-3-methylpyridinium bis((trifluoromethyl)sulfonyl)imide hinges on scaling production, fine-tuning purification, and closing the loop on environmental impact. Chemists keep hunting for cheaper routes, perhaps from renewable precursors, to cut costs and make this compound viable beyond boutique research. Industries eye performance boosts in energy storage, catalysis, and separations, but regulatory and waste management standards will shape how widely this ionic liquid sees real-world use. Recyclability and degradability matter more each year—to advance, research must deliver real solutions on both fronts. I see hope in pilot programs recovering and reusing ionic liquids from electrochemical processes, proving that practical circularity isn’t just a distant dream.
Tackling Tough Chemical Challenges
This long chemical name—1-Hexyl-3-Methylpyridinium Bis((Trifluoromethyl)Sulfonyl)Imide—might never roll off the tongue, but it’s well-known in labs and industries with complex tasks. This compound stands out as part of the ionic liquids family. Unlike water or standard organic solvents, it won’t turn into vapor at room temperature, and that means less risk of inhaling fumes or losing material to evaporation during careful procedures. Safety in my chemistry days was always about cutting down these hidden dangers.
Setting the Pace in Green Chemistry
Industrial processes often trigger headaches about what happens to waste. Traditional solvents, especially in pharmaceutical and specialty chemical plants, end up as hazardous leftovers. This ionic liquid has earned attention not just because it dissolves many things well, but because it resists breaking down or catching fire. According to several papers in journals like Green Chemistry and Chemical Reviews, replacing volatile organic solvents with alternatives like this often leads to lower emissions and easier containment of toxins. I remember the first time my team swapped out regular acetone for an early ionic liquid in an extraction—it felt like a breath of fresh air, literally, and it shifted the mood about what sustainable chemistry could be.
Boosting Battery and Electrochemical Tech
Modern batteries rely on electrolytes—the substances carrying ions from one electrode to another. Engineers constantly hunt for blends that support stable, long-lasting current flow. This ionic liquid shows up in research for lithium-ion and other rechargeable batteries. Its thermal stability means it keeps going strong even after long use. According to the Journal of Power Sources, cells using this substance as part of their electrolyte can reach longer life spans than those with more traditional liquid salts. Scientists often highlight its low volatility and wide electrochemical window—key features when prolonged charging or discharging cycles push ordinary materials past their limits. As someone who’s spent hours struggling with electrolyte leaks and broken seals, noticing a solvent that sticks around without wrecking the hardware counts as a real plus.
Catalysis and Complex Synthesis
Catalysts transform chemicals quickly and efficiently, but many traditional set-ups create waste or demand harsh conditions. This ionic liquid helps solve some of those bottlenecks. In transition metal catalyzed reactions, for instance, it can stabilize metal nanoparticles, keeping reactions humming longer and cleaner. Once a friend working at a specialty polymers firm shared their progress after swapping conventional solvents for this ionic liquid—runs went faster, yields jumped, and side products dropped. Evidence also hints at easier product separation compared with traditional methods, making purification less of a headache.
CO2 Capture and Environmental Remediation
Climate-focused researchers test all kinds of absorbents for grabbing up carbon dioxide. This compound’s structure gives it a knack for dissolving gases, especially under tough industrial conditions. Direct air capture and gas stream scrubbing projects have cited this ionic liquid as a tunable, less energy-intensive way to trap CO2 compared to amine-based systems. For those thinking beyond smoky chimneys, this approach could mean more adaptable, less costly carbon reduction.
Pushing Toward Safer and Smarter Materials
There’s growing movement for smarter, greener formulas in every area from electronics down to cleaners and lubricants. This compound fits well for advanced coatings, anti-static layers, and specialty lubricants, standing strong in extreme cold or heat and resisting break-down over time. Seeing a material that can make products more durable and stable, while reducing toxic output, gives hope for better practices where chemical safety and performance are both on the line.
Why Chemical Structures Matter
Science textbooks often show diagrams of molecules as tiny clusters of letters and sticks. It’s tempting to assume those sketches only matter to chemists in white coats, but these shapes and formulas decide much of what touches our lives. Take caffeine in your morning coffee, for example—the way its atoms knit together brings the energy that wakes people up. If the structure changed, you wouldn’t get the same boost. Anyone who has struggled to read the back of a medicine bottle or wondered about what’s really inside a cleaning product knows how confusing these chemical codes can appear. Learning the basics of formulas unlocks a new understanding of the world around us.
Breaking Down the Pieces
Chemical formulas lay out the elements and the count of each atom in a compound. H2O tells you that water uses two hydrogen atoms and one oxygen. Simple, but insightful—the same recipe found in every ocean, raindrop, and kitchen tap. A structural formula, on the other hand, shows how the atoms connect. In the case of water, drawing those little lines for bonds brings the picture to life, clarifying how hydrogen and oxygen combine. This information doesn’t just satisfy curiosity. It helps weed out confusion between similar-sounding substances, such as ethanol (C2H5OH, drinkable alcohol) and methanol (CH3OH, poisonous). One wrong atom and the effects shift from relaxation to real harm.
Real-Life Impacts of Getting It Right
Mistakes in reading or writing a formula can cause the sort of trouble that makes headlines. Pharmaceutical labs spend millions and run thousands of checks because even a tiny error can mean major health risks. The Food and Drug Administration has highlighted cases where confusing chemical structures slipped between safety barriers, leading to contaminated medicine batches. In schools, teens tasked with learning these formulas for exams need real-world examples to make the topic stick. Breaking apart the jargon for students helps nurture the next wave of scientists.
Solutions Worth Considering
Chemistry software and open databases offer more tools now than ever before. Open-access programs like ChemSpider and PubChem allow anyone to type in a compound’s name and see both structural diagrams and chemical formulas. A clearer digital record stops mix-ups, backs up quality control, and gives students a lifeline for homework frustrations. App developers now craft interactive molecule builders that help young learners visualize how atoms bond. These tools also help researchers cross-check new findings so friction and confusion drop, promoting safer product development.
Teaching should use real-life examples: showing students how pharmaceutical companies rely on precise formulas to make safe medicine, or how incorrect formulas led to famous industrial accidents. Public health campaigns can add more accessible graphics explaining what these formulas mean, cutting through scientific jargon and helping families make sense of food ingredients, cleaning product labels, or environmental news reports.
Moving Forward Through Clarity
Chemical structures and formulas shape the world in tangible ways. These combinations decide the taste, safety, and effectiveness of what people eat, drink, and use to care for their families. A little more public knowledge goes a long way toward safety and informed choices. Let’s keep building on today’s tools and lessons, making chemistry less of a secret code and more of a common language.
The Real Story on Product Safety
Most folks expect safety information to be clear as day, especially when it comes to products in your hands or around your family. I've handled my fair share of gear in workshops and at home, and I’ve learned nothing beats direct, easy-to-follow advice. You want to know: Can I touch this? Will it harm my skin? Does it need special storage? Overcomplicated technical talk only gets in the way.
Why Accurate Safety Info Matters
One botched warning can land someone in the hospital. Take paint thinners or powerful glues, for example. I’ve seen people work with these and skip gloves, thinking “It’s just a quick fix.” Hours later, they’re red and itchy or worse. Facts back this up: the American Association of Poison Control Centers receives thousands of calls every year about chemical exposures at home.
If the product contains substances like strong acids, solvents, or any compound flagged by the EPA, you’d better believe serious precautions come next. Labels aren’t there for show. They lay out steps based on years of nasty incidents and science. A friend of mine, an ER nurse, says most household accidents involve products you find under the sink or in the garage, not just the obvious stuff like pesticides or drain cleaner.
Basic Safety Guidelines Worth Following
Whenever possible, read labels. Don’t skip the fine print or rely on your memory. I recommend always having gloves when dealing with chemicals—disposable nitrile or heavy rubber work better than old cloth gardening gloves. Washing your hands thoroughly afterwards should become habit, even if your skin looks clean.
Ventilation also saves headaches and worse. If you can smell fumes, that tells you lungs are already on the line. Opening windows and running fans helps, but for strong vapors, respirators with the right filter are no joke. The CDC recommends them for more than a dozen home and work products often thought of as harmless.
Eye protection is often neglected. Splashes happen in a split second. Simple safety glasses prevent days of pain or permanent eye damage. A friend once skipped his goggles handling pool chemicals; his eye burned for a week despite fast rinsing.
Safe Storage and Disposal Practices
Don’t stash chemical products near kids or pets. Lock them up high or in cabinets with real closures, not just flimsy latches. Sunlight changes some chemicals, turning them toxic or explosive. Heat is also a no-go: garages get hot, bottles can swell or leak, and then you’re mopping up trouble.
Always check local waste management rules before dumping leftovers. Many communities run hazardous waste drop-offs; you don’t want these substances in the trash or down the drain. Everyone in the house should know the emergency number for poison control, because reacting quickly saves lives or permanent injury.
What Makes a Product Truly Safe?
Manufacturers carry responsibility, but in the end, so do we as users. While some companies cut corners, many offer detailed sheets (Safety Data Sheets or SDS). These lists aren’t just for factories—they make sense for home users too. Reading them answers the big questions: Is it flammable? Does it need special handling or storage? What first aid works best if something goes wrong?
Relying on facts and experience helps more than trusting intuition alone. Pay attention to the real warnings, not just what you remember hearing growing up. Small steps—like labeling bottles after you pour, using the right gloves, or storing supplies out of reach—make a world of difference.
Understand What’s at Stake
A lot of folks overlook just how critical storage can be for the products they count on. Whether we’re talking about medicine, food, chemicals, or even common household products, the wrong approach can turn something helpful into a liability. I learned this firsthand long ago, watching a shipment of carefully made supplements lose all potency because someone tucked it away in a sweltering warehouse. That single mistake wasted months of work.
Putting Safety First
Most products don’t react well to big swings in temperature or high humidity. Medicines, for example, can break down in the heat. Cosmetics might separate or start to smell off. I’ve seen warehouses that choose cost-cutting over climate control, and it rarely ends well. One bad batch and people start losing trust—sometimes for good reason.
Light poses its own risks. Vitamins like C or B2 degrade under bright light. Even paint in a sun-filled workshop can dry out or clump before it’s ever opened. So manufacturers use dark bottles or opaque boxes not just for looks, but to shield what’s inside.
The Science Backs It Up
Stability testing isn’t a suggestion—it’s a must. The World Health Organization and governments set clear guidelines. Drug manufacturers run tests for months or years, keeping samples in different conditions and measuring any changes. These studies do more than meet a rulebook; they stop dangerous recalls and protect public health. Take insulin. Store it at room temperature for a week, and it still works. Leave it out for a month, exposed to summer heat, and doses might suddenly fail to control blood sugar.
Packaging: More Than a Box
Packaging keeps oxygen, water, and light from spoiling a good product. I’ve seen producers change from plastic to glass simply because the old jars let in too much air. Even a little moisture can trigger mold growth in foods or degrade powders. A moisture-absorbing packet can make all the difference.
Keeping Track
Labels matter. Expiry dates, storage tips, and even warning symbols can change the game. I once bought a probiotic that worked wonders—until I forgot it in my car on a hot August weekend. The box did warn me, but I hadn’t paid attention. Clear guidance helps avoid those slip-ups.
Smart Solutions
Reliable refrigeration, dry storage rooms, or even simple airtight containers save money and prevent trouble. I tell friends with small businesses that even a cheap data logger—just a gadget tracking temperature and humidity—can pay off many times over. Local farmers use clay coolers that run without electricity. Pharmacies rely on backup power to keep medicines stable during outages.
Loss from poor storage doesn’t just hurt companies. It impacts customers, sometimes seriously. Folks deserve to know the products they buy are still safe and doing the job they promised. Setting and sticking to strong storage guidelines keeps everyone healthier and builds trust that can outlast any quick sale.
Looking Deeper Into Material Choices
Battery technology often drives progress in electronics, energy storage, electric vehicles, and more. The search for better performance often brings up questions about whether a newly-discovered compound could unlock longer-lasting batteries, faster charging, or safer use. Every time I read about a fresh compound making the rounds in research circles, I think back to the years spent troubleshooting lithium-ion cells in the lab. It’s the same question every time: does this new material offer any real improvement, or does it only look good on paper?
The Need for Practical Evidence
Researchers have explored crystal structures, ion conductivities, and thermal stabilities for decades. A compound might shine in a controlled environment, but out in the field, reality gets in the way. Certain materials break down after a few dozen charge cycles. Some react badly with moisture from the air. Others form dendrites—metallic whiskers that punch through and kill the cell. In battery work, even a tiny flaw grows into a full-blown reliability problem over thousands of cycles.
I remember handling anodes with promising theoretical energy density, thinking the numbers looked unbeatable. Yet, after repeated assembly and teardown, the electrolyte would swell, the coating cracked, and the cells never met expectations. That real-world trial and error roots my skepticism. It takes more than attractive data from the bench; the material has to survive tough abuse.
Safety and Environmental Impact
Every material introduced brings safety implications. Lithium cobalt oxide, for example, once looked like the perfect cathode. Its high energy density helped power everything from smartphones to medical gear. Then came reports of fires and environmental hazards. A new compound must not only store more charge, but also avoid toxic byproducts, runaway reactions, and tricky disposal problems. Regulations get stricter every year. Labs run dozens of extra tests, like thermal runaway evaluations and toxicity screens, before thinking about scaling up. None of the fancy numbers matter if the material can’t meet safety rules or raises risk for workers.
Fitting Into Existing Production
Major advances often come from incremental fit, not total replacement. Large manufacturers hesitate to overhaul their entire production line for one new ingredient unless the gain far outweighs the cost. In my time working with battery prototypes, the best ideas came from tweaking the binder or adjusting the electrolyte composition, slotting a new additive into an existing slot. Sometimes, a compound that works wonders in a coin cell doesn’t scale—too rare, too expensive, or hard to process in tons per month. Any new candidate must marry promise with practicality and affordability.
Pushing Toward Open Collaboration
The top research groups share data openly, compare findings, and replicate each other's work before making big claims. Open science helps sort hype from genuine utility. Recent trends see startups, universities, and established companies pooling resources to test samples in a variety of real conditions. The process weeds out flashy but flawed materials early and speeds up the path for compounds that truly bring something new to the table. Every cycle, every failure, and every breakthrough in these shared spaces benefits the whole field.