1-Hexyl-3-Vinylimidazolium Bromide: An In-Depth Look
Historical Development
Back in the late 1990s and early 2000s, chemists around the globe began searching for greener solvents. Ionic liquids offered something new—not just one, but hundreds of molecular options. 1-Hexyl-3-vinylimidazolium bromide (HVIMBr) started to attract attention because of its hybrid nature, blending an imidazolium core with both alkyl and vinyl groups. Researchers saw that the traditional chloro-alkane solvents were falling out of favor due to health and environmental worries. So, HVIMBr entered the stage, not as a copycat, but as a molecule that could take part in polymer chemistry, catalysis, and even electrochemistry. Its story runs parallel to the rise of green chemistry itself, shaped by practical lab needs and regulations pushing for safer, more sustainable materials.
Product Overview
In standard laboratory terms, HVIMBr comes as a pale yellow or clear, viscous liquid. The presence of a vinyl group gives it a unique handle for further reactions—unlike the ordinary alkyl-imidazolium salts that just act as solvents or electrolytes. You might spot it in a brown glass bottle, sealed tight, with hazard markings signaling sensitivity to moisture and the need for careful storage. Chemists pick HVIMBr for its ability to bridge two worlds: ionic liquid behavior and active functionality. Its dual use, as a solvent and a reactant, gives it special meaning in both industrial and academic laboratories.
Physical & Chemical Properties
HVIMBr features a melting point below room temperature and resists decomposition under gentle heating. Its imidazolium ring, linked with a long hexyl chain, makes it particularly hydrophobic compared to shorter-chain analogs. Yet, the bromide counterion imparts enough polarity to let it dissolve salts and organic compounds that ordinary solvents wouldn’t touch. Density typically hovers just above 1 g/cm³, and with a viscosity higher than water, HVIMBr moves slowly in the bottle—a sign of strong ion-pair interactions. The vinyl group at position three of the ring isn’t just decorative; it’s reactive, making room for addition, polymerization, or modification directly on the solvent scaffold.
Technical Specifications & Labeling
Pure HVIMBr, as sold, generally arrives with an assay above 98%. Labels call out sensitivity to light and air, with a suggested storage temperature below 25 °C. Common labeling includes the molecular formula (C11H19BrN2) and cautions for skin and eye contact. Chemical suppliers apply unique catalog numbers, product codes, and safety data sheet links. Clear labeling supports safe transfer and minimizes chances for mix-ups. In multi-language packaging, warnings stay front and center, following the Globally Harmonized System (GHS) for hazard communication. Please remember: thorough reading of safety data well ahead of the first use remains a non-negotiable step.
Preparation Method
The classic approach starts with 1-vinylimidazole, itself a reactive building block, combined with 1-bromohexane. The reaction proceeds best in anhydrous polar solvents—acetonitrile or acetone stand out—as water can cause hydrolysis or lower yields. Gentle heating, usually below 80 °C, prompts nucleophilic substitution, letting the hexyl group attach to the nitrogen on the ring. Patience pays off, as the process sometimes stretches overnight. Purification, crucial for research-grade stock, calls for repeated washing with ethyl acetate and sometimes vacuum drying to strip off all volatile residues. Quality control by NMR, IR, or sometimes even mass spectrometry, verifies that every bottle contains the right product and nothing unwanted.
Chemical Reactions & Modifications
The vinyl group unlocks a range of chemical tricks. Polymer chemists covet HVIMBr for living radical polymerizations, where it acts not just as monomer but as a medium. You can build elaborate copolymers by combining HVIMBr with acrylates or styrenes, embedding ionic functionality right along the polymer backbone. Bromide plays its part, too—as a potential leaving group or as a counterion swap candidate. Exchange with other anions like bis(trifluoromethanesulfonyl)imide pushes the material toward applications in advanced electronics, membranes, or battery electrolytes. Both the cation and anion can change through straightforward synthetic procedures, opening pathways toward libraries of related structures with subtly different behaviors.
Synonyms & Product Names
While catalogs stick to the systematic name, chemists sometimes shorten the handle to HVIMBr or even simply “vinylimidazolium bromide.” Under various suppliers, you might see it listed as 1-hexyl-3-vinylimidazolium bromide, C11H19BrN2, or with proprietary names like “functionalized imidazolium ionic liquid.” There’s no industry-wide agreement on shorthand, so double-check before ordering to avoid landing the wrong bottle on your bench.
Safety & Operational Standards
HVIMBr demands practical respect in daily handling. Its moderate toxicity means full PPE in the lab: gloves, goggles, and lab coat. Even though it doesn’t give off much vapor, skin contact or swallowing can pose health risks. Disposal follows local chemical waste regulations, as releasing ionic liquids into water streams or landfill harms aquatic environments. The bromide content in particular raises red flags due to potential reactivity under strong acids or oxidizers. Proper ventilation trumps shortcuts, and all containers get sealed tight after use. Regular risk assessments, never just for new users, become habit over a long career with these materials.
Application Area
With HVIMBr, possibilities run wider than just “green chemistry.” In organic synthesis, the vinyl function allows direct participation in reaction networks, switching from solvent to reagent simply by adjusting the stoichiometry. Analytical chemists take advantage of its solubilizing powers for tricky separations and as a liquid-phase extraction medium. In materials science, HVIMBr forms ionic polymers or hybrid membranes, boosting transport properties and mechanical toughness. Battery researchers lean in on the low volatility, stability, and conductive nature of this class. It finds occasional use in textile treatments thanks to strong fiber interactions, and even crops up in enzyme immobilization—showing how the field keeps finding new roles for this versatile ionic liquid.
Research & Development
Right now, labs worldwide pursue ways to expand the functional group chemistry possible with HVIMBr, both at the academic and pre-commercial stages. Polymer scientists explore its use in block copolymers, designing solid or gel electrolytes that might replace less stable equivalents in electronics. Electrochemical device developers test its ability to carry charge in supercapacitors, fuel cells, and sensitive humidity-sensing devices. Teams in analytical chemistry treat HVIMBr as a customizable extraction tool, targeting everything from heavy metals to pharmaceuticals in environmental or food samples. Each discipline faces hurdles—such as cost per gram, stability outside controlled environments, or ease of recycling—but the ongoing work reflects broad curiosity and confidence in future payoffs.
Toxicity Research
Like many ionic liquids, HVIMBr’s environmental and toxicological profiles still need deeper understanding. Early studies flagged moderate toxicity to aquatic life, mainly due to the bromide anion and the persistent nature of imidazolium structures. Mammalian studies suggest low acute toxicity, yet chronic exposure over months or years has not been fully mapped. The hexyl side chain may add bioaccumulation risks by facilitating transport through biological membranes—raising tough questions about downstream impacts if large-scale manufacturing takes off. Environmental scientists call for tighter regulation and lifecycle analysis, advocating for better waste treatment and reusability in industrial settings.
Future Prospects
Looking forward, HVIMBr stands to attract greater interest in the contexts of circular chemistry and advanced manufacturing. Efforts to integrate real-time monitoring and rapid purification could bring down costs, meaning expanded access for research and industry alike. Polymer design, ion conduction, and even pharmaceutical synthesis stand to benefit from new generations of tailored ionic liquids—products that do more than merely replace old solvents. The challenge will stay the same: maintaining performance while protecting workers and the environment. For HVIMBr, continued collaboration between chemists, engineers, toxicologists, and regulators could shape a future where such advanced materials see everyday, safe, and responsible use across many industries.
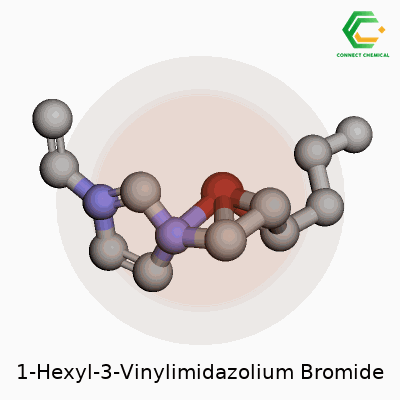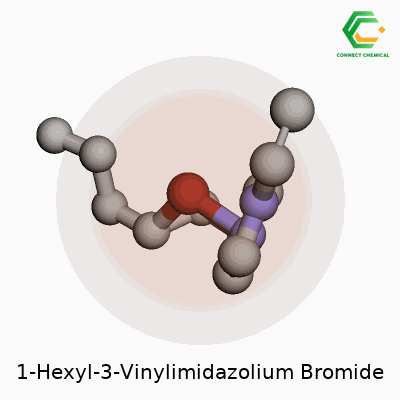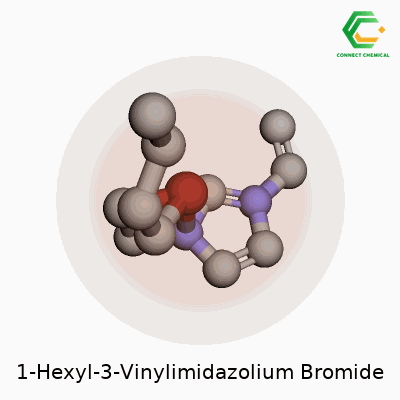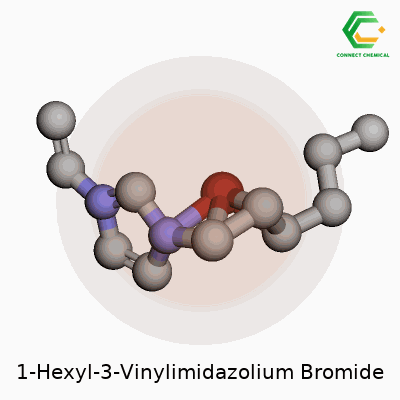
Decoding the Backbone: Why Structure Has a Story
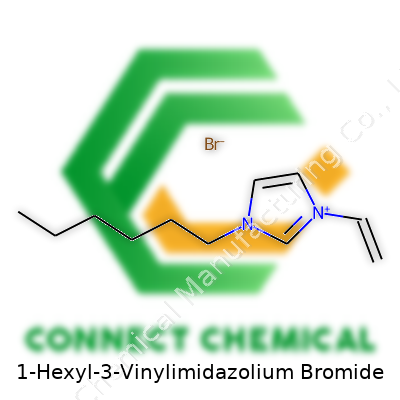
Every molecule tells a unique story through its structure. 1-Hexyl-3-vinylimidazolium bromide shows this better than most. This compound features an imidazolium ring, with two special attachments: a hexyl group and a vinyl group. Put the pieces together and you have a backbone built for flexibility and reactivity. The hexyl tail gives the molecule a non-polar, grease-friendly part. The vinyl group, sitting on the imidazole, brings the option for further chemical reactions—think of adding handles for new uses. Then there’s bromide, the counterion that balances charge, grounding the molecule and shaping properties from melting point to solubility.
Drawing the Structure: Atoms, Bonds, and Branches
Chemically, you’ll see a five-membered imidazole ring, two nitrogen atoms, a double bond in the backbone. One of these nitrogens has a hexyl chain—the six-carbon, straight tail. Another nitrogen wears a vinyl group, a two-carbon chain ending in a double bond. Add the positive charge sitting on the ring, thanks to the extra binding from those groups. To finish, a loose bromide anion clouds nearby, balancing out the positive charge of the imidazolium core.
It’s not just an academic drawing. Picture it in the lab: white crystals, maybe a little sticky depending on moisture, but far from the simple salt you’d sprinkle on fries. The bonds and ring shape make sure each property—solubility, reactivity, and thermal stability—line up in a certain way. People in the lab count on this predictability.
Function Follows Form: Why This Structure Earns Its Place
What makes this molecule stand out isn’t just a pretty diagram. The imidazolium ring, known for stability, resists breaking down under heat or chemical stress. Hexyl boosts solubility in organic solvents and even changes how the whole molecule slips into polymers or mixes in ionic liquids. The vinyl arm doesn’t just sit around; it gives a reactive spot for linking into polymers or networks. Each part of the molecule comes together, making it a favorite in the toolbox of researchers building new materials or batteries.
From my experience watching battery research, molecules like 1-hexyl-3-vinylimidazolium bromide show up in places people don’t always expect. Ionic liquids built from this structure handle electricity like champs while shrugging off water and air. That stability means less worry about fires or leaks—something any chemist or engineer can appreciate. The vinyl group means you can weld the molecule right into a new structure, anchoring it so it doesn’t wander off. It’s an approach that saves time in the lab and boosts the performance of the final product.
Looking Ahead: From Structure to Solution
Designing better materials starts with picking the right building blocks. The hexyl and vinyl attachments stretch the imidazolium’s potential, solving problems around conductivity and durability. If industrial labs want to reduce toxic solvents and boost battery lifespan, turning to molecules like this one makes sense. The well-chosen side chains and reliable ring help researchers sidestep common headaches: breakdown under stress, weak conductivity, or issues mixing with other ingredients. Tuning these molecular features leads directly to safer, longer-lasting, and more adaptable products.
Building new solutions depends on understanding these structures inside and out. 1-Hexyl-3-vinylimidazolium bromide stands as proof that detailed chemistry can make daily products safer and better for everyone.
Behind the Name: What Makes This Chemical Interesting?
In the lab, a name like 1-Hexyl-3-Vinylimidazolium Bromide usually means business. It’s an ionic liquid, which means it stays liquid at room temperature unlike regular table salt or sugar. The combo of a hexyl chain and a vinyl group gives it a special personality compared to other ionic liquids. That unique structure pops up in some serious tech, not just in chemical textbooks or on whiteboards.
Electrochemistry: Power and Storage
Rechargeable batteries need engineers to push limits every year — nobody wants their phone dying after lunch. Ionic liquids like 1-Hexyl-3-Vinylimidazolium Bromide stand out for high thermal stability and almost zero vapor pressure. This matters if you’ve ever seen a battery bulge or catch fire. With this chemical as an electrolyte, batteries tend to last longer and handle higher temperatures. That contributes to safer, more dependable energy storage, whether in electric vehicles or big wind farms. Reports in the Journal of Power Sources show imidazolium salts letting lithium-ion batteries run at much higher voltages. Researchers at several universities point to fewer leaks and less flammability with these salts, keeping lab workers safer and gear working longer.
Green Chemistry Meets Industry
Traditional solvents, like acetone or toluene, have a bad rep because they evaporate and pollute the air. Large factories still use them, but that’s changing. 1-Hexyl-3-Vinylimidazolium Bromide offers a far greener route. This ionic liquid doesn’t just sit in a bottle; it dissolves polar and non-polar compounds alike, letting chemists skip the smelly, toxic stuff. This helps with recycling, lowers hazardous waste, and keeps the air inside labs and plants cleaner. Studies in Green Chemistry and the EPA’s green solvent guidance praise ionic liquids for similar reasons.
Smart Polymers and Advanced Materials
Ionic liquids change the game in making fancy plastics, coatings, and membranes. The vinyl group on this molecule takes part in polymerization—the basic process that makes something like a flexible phone case or a water purification membrane. Scientists blend it in with other building blocks to tweak flexibility, stickiness, or how well they let certain molecules pass through. From experience in the lab, adding an imidazolium-based ionic liquid can turn a sluggish polymerization into a smooth, controlled process, with fewer leftover chemicals and better quality in the final product.
Catalysis: Getting Reactions to “Go”
Many reactions grind to a snail’s pace or just don’t work at all in water. Toss 1-Hexyl-3-Vinylimidazolium Bromide in the mix, and suddenly chemists can push stubborn molecules together. It boosts reaction rates and delivers cleaner results, meaning less sorting out junk afterward—something any chemistry student will appreciate. It’s especially handy for alkylation, Diels-Alder, and Heck reactions, all cornerstones of drug and material synthesis.
What’s Next?
These days, the hottest research looks at recycling and circular economy. Ionic liquids like this one could one day let us reclaim rare metals from old electronics. Researchers in Europe have already used similar chemicals to strip gold and platinum from circuit boards without blasting out toxic fumes. Smarter batteries, cleaner chemistry, and new materials all depend on this class of chemicals. The more we explore them, the closer we get to everyday tech working better and doing less harm.
Working with Unpredictable Substances
Anyone who has spent time around a chemical bench knows what a mess things become when storage rules get ignored. Leaks, cross-contamination, and clouded labels mean trouble for the people around you and the quality of your work. Beyond that, accidents damage trust. Nobody wants to hear about someone ending up with burns or worse, just because a bottle ended up in the wrong cabinet or with the cap half screwed on.
Understanding What You're Handling
Chemicals bring a wide range of risks. Take something flammable—one spark, and it tries to set your world on fire. Corrosive substances eat through metal and skin faster than you’d believe. People think certain compounds stay stable as long as you seal them, but that’s only half true. Air, light, and even the kind of container can turn an everyday bottle into a hazard. I’ve seen glass vials shatter from pressure buildup, or simple negligence lead to fumes leaking into a cramped closet.
Eye on the Environment
Temperature has always played games with chemical stability. Some reagents turn volatile just sitting outside on a warm afternoon. I keep a separate fridge for certain compounds because mixing food and chemicals leads to poison in both. Moisture in the air messes with powders, clumping them up or triggering reactions you don’t want happening on a shelf.
Protecting Yourself and Your Crew
Simple habits go a long way. Gloves, goggles, and lab coats may seem like overkill, but skin and eyes don’t heal easily after contact with something caustic. Label everything like your future depends on it. It does. Ignoring expiration dates or missing hazard symbols create surprises you don’t need. I keep a folder of each material’s safety sheet right where supplies live, so nobody has an excuse for being ignorant.
Security and Accountability
Not every compound stays harmless if someone with ill intent gets their hands on it. Keeping storage locked and logging who takes what means fewer unaccounted vials. Clear records also let you track where mistakes pop up and prevent repeat accidents.
Disposal—Not Just an Afterthought
Disposing of leftover or expired material ranks among the most overlooked jobs in a lab. Dumping chemicals down the drain launches them straight into nature’s food chain. Special disposal bins exist for a reason. These cost a little extra time, but they keep your conscience and workspace clean. In places with strict environmental rules, improper disposal racks up fines and ruins community relations quickly.
Building a Culture of Safety
Spending my days in labs, I learned that every shortcut taken now leads to a bigger mess later. If you see a bottle on a warm shelf or a label smeared off by acid splatter, fix it before carrying on. Regular checks mean catching small mistakes before they become emergencies. Sharing tips, reporting near-misses, and sticking together keep everyone working, not just ticking boxes for regulations but making sure we all head home in one piece.
Simple Steps with Big Payoffs
Start with good ventilation and correct labeling. Separate incompatible groups by distance or physical barriers. Rotate old stock out for new, never stockpiling more than you actually need. Pay attention to training new people so they know exactly how to store, handle, and respond to spills. These steps mean fewer accidents, better research outcomes, and a safer working environment—including for the people who come after you.
Understanding Solubility: Real-Life Stakes
Picking the right solvent for a chemical like 1-hexyl-3-vinylimidazolium bromide isn’t just about ticking a technical box. In industries like pharmaceuticals or advanced materials, mistakes about solubility can mean wasted batches or even hazardous lab conditions. From my own experience working in research labs, nothing throws a wrench in progress like grabbing the wrong solvent. The result is clumped-up material that refuses to dissolve or a dissolved mess that’s impossible to purify.
The Science Behind Solubility
1-Hexyl-3-vinylimidazolium bromide belongs to a family called ionic liquids. These salts usually stay liquid at room temperatures. Their unique composition affects how they behave: the imidazolium ring brings a touch of polarity, but the long hexyl chain leans towards grease, and the bromide counterion cranks up the ionic charge. Here’s what the evidence tells us:
- This compound shows good solubility in polar and moderately polar organic solvents. Examples like methanol, ethanol, and acetonitrile typically work well—lab manuals and published reports back this up.
- Water solubility often exists but isn’t guaranteed. Smaller imidazolium-based salts (with tiny alkyl chains) dissolve freely in water, but as the alkyl group grows, water compatibility shrinks. The hexyl group here adds a hydrophobic push, so don’t expect it to completely mix with water the way table salt does. Sometimes, you’ll see partial or cloudy solutions.
- Chlorinated solvents or nonpolar organics (think hexane or toluene) usually don’t work. That’s because this compound still carries that ionic nature, which doesn’t sit right in oily environments.
Why Does It Matter?
Getting solubility right isn’t just academic: it keeps labs safe and projects moving. In the synthesis of ionic liquids for battery electrolytes—an area I’ve followed closely—solvent missteps can wreck reaction efficiency or contaminate the end product. There are also worker safety concerns; choosing a toxic solvent for convenience or speed isn’t ethical, especially if safer alternatives exist.
Backed by Studies and Industry Data
Look at the scientific literature: journals like Green Chemistry and Chemical Communications regularly publish data tables for solubility of ionic liquids. One 2018 study showed that imidazolium-based compounds with hexyl side chains dissolved completely in methanol and acetonitrile but only partially in water. Sigma-Aldrich and related chemical suppliers also flag these trends in their technical sheets. Trust and transparency depend on these real-world sources.
What Can Be Done?
If a process calls for this compound, smart choices reduce risk. Test initial dissolving in a small batch, logging results for water and common organic solvents. Reputable suppliers often outline recommended solvents, but don’t hesitate to ask for sample data or contact technical support. Colleges and companies should offer more training on reading solubility data—too many mistakes still happen from guessing or bad assumptions.
1-hexyl-3-vinylimidazolium bromide blends the worlds of ionic and organic chemistry. It takes careful attention to choose the right solvent, and the facts point toward safer, more consistent results with alcohols and acetonitrile. Water may help in some cases, but only testing and good data prevent frustration and danger.
Packing a Punch: How Purity Shapes Buyer Trust
Purity acts as more than just a benchmark in chemistry circles. Anyone dealing with raw materials quickly learns that purity holds a direct impact on a product’s usefulness. In pharmaceutical manufacturing, for instance, customers keep a sharp eye out for anything below 99%. A small drop in purity numbers can threaten entire production lines. Those working with food additives or supplements also expect nothing less than high standards—most vitamins and minerals ship at 98% or higher.
This attention to purity grows from years of hard lessons. I remember speaking with a plant manager whose output suffered from inconsistent supply. Their previous supplier provided a product labeled “98%+” but never shared COAs (certificates of analysis). When a batch came in at 95%, it threw off formulations, wasted time, and forced expensive do-overs. Their story isn’t unique. Having clear, verifiable purity keeps buyers safe from these headaches, and smart vendors disclose not just numbers but also test methods and batch-specific details.
Labeling and Transparency
Many seasoned buyers spot red flags in vague claims like “technical grade” or “industrial purity.” Reliable suppliers commit to publishing actual test results instead of relying on umbrella labels. These reports break down major and trace impurities. Analytical techniques—often HPLC or ICP-MS for high-value products—get cited right on the documents. Pharmaceutical and electronics producers often request purity as high as 99.9%, while more forgiving applications, like industrial cleaning, settle near 90% or even lower.
Honest labeling meets not only customer expectations but also regulatory demands. In Europe, for example, strict REACH guidelines limit certain contaminants. Purity information plays a key role in deciding if a product meets legal thresholds. As markets demand cleaner products, having open access to these details builds seller reputations and keeps both sides out of legal trouble.
Practical Packaging Sizes: Big Totes, Small Bottles, and Everything In Between
Product packaging tells its own story. Bulk industrial users often call for 25 kg sacks or 50 kg drums. These heavy-duty containers suit high-volume factories and come with sturdy linings to prevent leaks or contamination during shipment. On one of my site visits, I saw teams using forklifts to move pallets loaded with these bags straight from trucks to storage. In contrast, laboratories lean on bottles or pails from 100 grams up to 1 kilogram—manageable for careful dosing and small-batch experiments.
Shipping hazardous or sensitive materials brings another layer of complexity. Double-bagging, vacuum seals, or inert gas blanketing keep moisture and oxygen at bay for chemicals that spoil or oxidize easily. In the food and supplement world, regulation-driven packaging leads to tamper-evident seals, clear batch numbers, and even QR codes linking to lab reports.
Buyers switching between sources often prefer flexible sizes, especially during testing or pilot runs. Some suppliers started offering sample packs of 10 grams up to 250 grams as an answer to this demand. Simple adjustments like these save customers time and money, while easing long-term partnerships.
Moving Forward: Building Trust in Every Container
The marketplace rewards companies that treat purity and packaging details as more than a formality. Detailed reporting, open communication, and responsive packaging options help turn new customers into steady clients. Businesses earn trust by putting information front and center, not as a fine-print afterthought. After enough years working with procurement teams, I see how much smoother operations run with clear answers upfront—buyers sleep easier knowing the next shipment won’t hold any surprises.