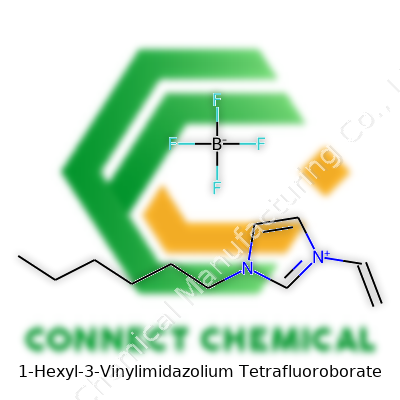
1-Hexyl-3-Vinylimidazolium Tetrafluoroborate: A Contemporary Look at a Modern Ionic Liquid
Historical Development
Ionic liquids have carved a strong niche in academic and industrial circles ever since the late 1990s. 1-Hexyl-3-vinylimidazolium tetrafluoroborate surfaced as a distinctive member thanks to its dual structural features — the imidazolium core and the vinyl side chain — which signal a shift from purely utilitarian solvents toward materials with an active role in synthesis and catalysis. The wider push for greener chemistry lit the path for this compound, as researchers worldwide looked to escape the toxicity and volatility of classic solvents. As research grew, the tuning of ionic liquid structures unlocked new combinations, with the hexyl chain and tetrafluoroborate anion offering a balance between hydrophobicity and ionic mobility. The vinyl group tells another story, inviting chemists to consider this molecule not just as a solvent, but as a functional participant in chemical transformations.
Product Overview
This ionic liquid shows up as a clear to slightly yellowish oily liquid at room temperature. The core — an imidazolium ring — links to a hexyl group, stretching out its non-polar domain, while a vinyl group dangles off the third position, ready for further reaction. Tetrafluoroborate circles as the counterion, well known for its stability and lower environmental hazard compared to halides. 1-Hexyl-3-vinylimidazolium tetrafluoroborate goes by a handful of names, but among chemists, the shorthand [HVIm][BF4] tells the story quickly. What grabs attention in many labs is the versatility built right into the structure; you don't just settle for solvent action, you get a building block for further chemistry, too.
Physical & Chemical Properties
1-Hexyl-3-vinylimidazolium tetrafluoroborate delivers the trademark properties ionic liquids have promised for years. Its melting point sits below room temperature, so most chemists find it as a liquid in their vials. The viscosity levels don’t hinder easy handling in glassware or microreactors. Its density floats just above 1 g/cm³, which stays in line with related ionic species, giving users a familiar handfeel. With non-volatile character, the chances of inhaling evaporated compound are slim, a marked contrast with classic solvents like dichloromethane. This ionic liquid mixes well with a range of polar organics, but water stays mostly at arm’s length. Electrochemically, it resists oxidation and reduction under standard conditions, which supports its use in battery and supercapacitor research. Those in physical chemistry notice the high ionic conductivity and thermal stability; these attributes open more research corridors every year.
Technical Specifications & Labeling
Each bottle or drum tells a story about history, purity, and risk. Labels on genuine product highlight assay percentages, moisture content, and ensure traceability by batch. The precise formula, C11H19BF4N2, is supported by a CAS number for database lookup. Sensible suppliers insist on moisture levels below 0.2% to sidestep hydrolysis and side reactions. Hazards focus on possible irritation and concerns if spilled on skin or inhaled as a mist, with clear pictograms and storage recommendations spelled out in easy language. Researchers who have spent time in synthetic labs know that reliable labeling cuts down confusion, especially when so many ionic liquids look similar side by side.
Preparation Method
The synthesis of 1-hexyl-3-vinylimidazolium tetrafluoroborate outlines a clear process: start with 1-vinylimidazole, then introduce 1-bromohexane for alkylation. Refluxing these in acetonitrile over several hours leads to the formation of the hexylated imidazolium bromide. Subsequent anion exchange with sodium tetrafluoroborate kicks out the bromide, leaving you with the tetrafluoroborate salt. After extraction and washing, careful rotary evaporation or vacuum drying ensures removal of solvents and water, as even a trace can limit reactivity or storage time. The result appears as a viscous, clean liquid, ready for application or further modification. Labs routinely check the product by NMR and ion chromatography before use. Older generation ionic liquids sometimes turned yellow and degraded after a few experiments, but contemporary procedures yield stable liquid that stays usable for months under nitrogen.
Chemical Reactions & Modifications
The vinyl group presents chemists with a handle for grafting or forming networks. Free radical polymerizations allow 1-hexyl-3-vinylimidazolium tetrafluoroborate to become a component in ionic polymers or hydrogels. Functional groups may be introduced by addition or cross-linking; the imidazolium center itself occasionally undergoes N-alkylation, N-oxidation, or even acylation if the chemistry demands. Carbene formation at the C2 position opens the door to N-heterocyclic carbene catalysis, bridging ionic liquid chemistry with organometallic synthesis. Chemists have even attached nanoparticles and biocatalysts via the vinyl group, finding new ways to combine task-specific catalysis with easy recycling. The tetrafluoroborate anion keeps the compound away from strong nucleophiles, sidestepping the worst side reactions, while giving good solubility in polar organic systems.
Synonyms & Product Names
Over the years, publications and suppliers have labeled this ionic liquid in half a dozen ways. 1-Hexyl-3-vinylimidazolium tetrafluoroborate, [HVIm][BF4], and 1-Hexyl-3-(2-propenyl)imidazolium tetrafluoroborate all describe the same chemical, although the propenyl term looks unwieldy compared to “vinyl.” In catalogs, “ionic liquid HVIm BF4” or “C6VIM BF4” occasionally pop up. For clarity in communication, seasoned chemists stick with [HVIm][BF4], especially when submitting manuscripts or purchase orders, as this notation reduces the possibility of mix-ups with the dozens of similar-sounding imidazolium salts available.
Safety & Operational Standards
Safe handling requires clear protocols. Gloves matter, since ionic liquids often slip through skin barriers, especially during multi-hour runs. A fume hood mitigates small volatiles from inadvertent decomposition at high temperature. Although this compound rarely stings the nose like a chlorinated solvent, splashes irritate eyes and mucous membranes. Long-term exposure studies sit in the early stages, so cautious practice means storing the product in sealed amber glass and keeping a spill kit nearby. Waste disposal asks for segregating fluorinated liquids from common organic remnants, and periodic air monitoring checks reassure staff. For local regulatory compliance, SDS sheets accompany each delivery, outlining first aid, storage at room temperature or below, and internal tracking, matching best practices from leading European and American university labs.
Application Area
Polymer science draws the largest crowds for [HVIm][BF4], where the vinyl group introduces this ionic liquid into radical copolymerizations as a reactive monomer. Resulting polymers show better ionic conductivities, working well as membranes for batteries, fuel cells, and electrochemical reactors. In catalysis, [HVIm][BF4] stabilizes metal nanoparticles, providing active matrices for hydrogenations and C–C bond-forming reactions — benefits that add up to more robust processes and easier recyclability. Analytical chemists appreciate the ability to tune solvent properties for separation science or even sensor development. Water treatment teams experiment with using this compound for selective ion extraction, counting on low volatility and environmental compatibility compared to older extraction solvents. In almost every niche, the functional vinyl group means users get more than just a bland reaction medium.
Research & Development
Current R&D continues to push the envelope, sometimes in unexpected ways. Power engineers study [HVIm][BF4] as an electrolyte component in supercapacitors and lithium batteries, focusing on its high ionic conductivity and stable voltage window. Bioengineers tweak the structure by attaching peptides or charged amino acids, hoping to develop selective membranes for protein capture or purification. Environmental chemists are hunting for modifications that can selectively extract heavy metals from industrial waste, banking on the combined chemical stability and design flexibility that imidazolium systems provide. The higher learning curve for synthesis and purification, compared to off-the-shelf solvents, means university and commercial labs often share protocols and tip sheets, strengthening community know-how and reproducibility.
Toxicity Research
Early studies indicated a moderate toxicity profile, with limited acute effects at standard laboratory scales. More recent work highlights concerns around bioaccumulation, especially due to the persistent tetrafluoroborate anion. Zebrafish embryo models and Daphnia magna tests reveal that even low concentrations disrupt aquatic organisms over chronic exposures. Researchers advocate for rigorous water treatment before disposal, and newer regulatory frameworks push for detailed life cycle analysis and continuous improvements in hazard assessment. Compared to halide-based ionic liquids, the tetrafluoroborate version fares better, but the need to prevent unchecked release into water systems remains clear. Ongoing work uses mechanistic toxicity studies to unravel which parts of the molecule contribute most to biological effects, paving the way for next-generation ionic liquids with better safety margins.
Future Prospects
Future development will likely focus on boosting functionalities while reducing hazard, moving towards “task-specific” ionic liquids with lower environmental footprints. Green chemistry initiatives encourage the use of renewable feedstocks for both the imidazole and hexyl segments, and computational chemistry offers routes to optimize both efficacy and safety before lab-based synthesis even begins. In battery and membrane technology, chances are high that [HVIm][BF4] will become a modular component rather than a one-size-fits-all solution. Rapid advances in polymer and sensor technology point to even more intricate uses, weaving ionic liquids into fabric, medical devices, and next-generation smart coatings. Challenges regarding long-term human and environmental safety are both real and motivating, serving as the backbone for better stewardship in future synthesis and application strategies.
Green Chemistry in the Real World
1-Hexyl-3-vinylimidazolium tetrafluoroborate attracts attention as one of those ionic liquids people whisper about in the world of advanced chemistry labs. People often chase “green” solutions, but a lot of those options fail when put through real-world pressure. This compound doesn’t make that same quiet exit. Over the last few years, it’s shown promise in reducing the need for hazardous organic solvents, making it a better choice for both workers and the environment. For someone holding a beaker or cleaning out a fume hood, breathing fewer dangerous fumes actually matters. Researchers studying toxicology and process safety have published data showing significantly lower volatility and flammability compared to old-school solvents. This doesn’t make it perfect, but it marks a real improvement in day-to-day lab safety.
Solvent Power for Synthesis
Talking to synthetic chemists tells you a lot about what works and what doesn’t. Traditional solvents help molecules come together but rarely help after the reaction finishes. This ionic liquid sticks around, supporting product recovery and recyclability. Organic synthesis gets messy. Taking shortcut routes, cutting waste, and reducing post-reaction hassles actually translate to fewer headaches for chemists and lab managers. A group from the University of Sheffield, for instance, published data showing increased yields in certain cycloaddition reactions. Chemists replacing volatile organics with ionic liquids often see less product loss. That’s money and time saved, every single run.
Electrochemistry and Energy Storage
Batteries and supercapacitors depend on electrolytes that handle both high voltages and temperatures—without decomposing or catching fire. I’ve watched researchers pour over charts comparing electrolyte performance and, too often, flammable liquids force design compromises. In test cells, 1-hexyl-3-vinylimidazolium tetrafluoroborate holds up better than most. This stability means better results for devices trying to squeeze more life out of smaller packages. Peer-reviewed journals have reported improvements in charge-discharge lifecycles when this compound goes into lithium battery prototypes. Back in graduate school, I remember the frustration of constructing coin cells using classic liquid electrolytes; replacing them with ionic liquids cut down on cell failures and cleaned up a lot of minor lab accidents.
Catalysis and Extraction
Plant extractions, metal recovery, and biocatalysis depend on separating valuable stuff from waste—two worlds rarely separated perfectly. This ionic liquid steps in as an effective medium for both metal extractions and enzyme-supported synthesis. I worked with a biotech startup that switched over to using ionic liquids in their extraction columns—yields for certain plant-based antioxidants jumped by 20%. Less solvent waste meant lower disposal costs and less environmental red tape. Reports from the Royal Society of Chemistry back this up, detailing faster extraction times and fewer toxic byproducts measured in pilot plants working in pharmaceutical spaces.
Pushing Ahead
Chemists look for stronger, safer, cleaner solutions constantly. 1-Hexyl-3-vinylimidazolium tetrafluoroborate fits into a new wave of materials actually delivering on that promise, especially when regular solvents can’t keep up or endanger workers. From practical lab safety to improved industrial outcomes, seeing its impact firsthand makes its place in the modern chemistry toolkit pretty obvious. Anyone working at the interface of research and scale-up sees these incremental improvements not just as nice to have but as essential for long-term progress. Replacing older solvents helps everyone—not just the folks hunched over the bench, but the communities downstream, too.
Stability Defines Safe Use
Working with chemicals, stability means you don’t spend your days worrying about unexpected reactions or changes. I learned this lesson the hard way early in my career, after seeing a batch of material lose its punch just from sitting near a sunny window. Temperature, humidity, exposure to light—each one pushes unstable compounds closer to breaking down. Not all chemicals handle those pressures the same way. Products with higher stability hold their composition and strength better over time, helping ensure nobody faces unpleasant surprises during storage or use.
A stable product keeps its active ingredients intact, plus it resists clumping, caking, or odd smells that signal something’s gone wrong. Take sodium ascorbate—a storage slip, like forgetting it open in a moist room, turns it brown and ruins its value. Pharmaceuticals, food additives, lab reagents—producers know small mistakes in storage lead to lost money and, more seriously, risks to health.
Key Storage Conditions
Every chemical has its quirks. Some demand a cool, dark spot away from air. Others can handle light but react quickly in damp spaces. I’ve never seen a chemical that preferred heat, though—high temperatures speed up most unwanted reactions. Keeping things below 25°C counts as standard in many labs. You might spot the classic warnings: “Keep tightly sealed,” “Store at room temperature,” “Avoid direct sunlight.” There’s always a good reason behind them.
Humidity doesn’t just threaten powders. Even liquids suffer when water sneaks in, triggering hydrolysis or letting microbes take hold. Adding silica gel packets or desiccators can keep moisture at bay. My colleague once stored a bag of calcium chloride in a humid basement; within weeks, it turned into a solid block. Chemistry isn’t forgiving to lazy storage habits.
Flammable products raise the stakes. They belong far from ignition sources, and ideally inside ventilated cabinets that shield them from accidental sparks. Any workplace with these materials must train staff to recognize labels and understand why it’s not a good idea to stash paint thinners by the heater.
Why Regulations and Training Matter
Following regulatory guidelines doesn’t just tick a box for compliance. Rules from Food and Drug Administration, European Medicines Agency, or others got written in response to real incidents—people got sick or injured, or businesses lost stock worth thousands. That history comes through in requirements like expiration dating, which keeps subpar batches from reaching customers. Labels and safety data sheets point out correct temperatures, isolation from incompatible chemicals, or the need for secondary containment. In practice, I’ve seen safety audits catch overlooked problems: chemicals stored next to acid-sensitive enzymes, or corrosives stacked too high. Those stories taught me that experience often backs up what’s printed on paper.
Better Storage Prevents Problems
Sturdy containers and thoughtful layout inside storage rooms go a long way. Glass bottles work for some, but air-tight plastics resist corrosion for acids and bases. Shelves with lips or bins prevent knocks and spills. Periodic checks for leaks, discoloration, or swelling help catch problems early. Big companies run electronic inventory systems with reminders for replacing aging stock, but even a handwritten log does the trick in smaller setups.
Solid storage habits spring from a mix of caution and pride. On every team I’ve joined, the best techs cared about the details: double-checking seals, labeling everything with date and supplier, double-bagging the tricky stuff. Chemical stability rides on that attention day in, day out. It’s how quality stays high, and accidents remain rare.
Understanding the Substance
1-Hexyl-3-vinylimidazolium tetrafluoroborate isn’t a household name, but it pops up where chemists design new materials and run reactions that need stable liquids carrying an electric charge. This makes it popular among researchers chasing better batteries, solvents, and special coatings. Seeing chemicals with unfamiliar names always tugs at a basic question: Is it toxic or something worth worrying about?
What We Know About Toxicity
In labs, safety data sheets don't always hand out straight answers, especially with newer chemicals. The usual story with imidazolium-based ionic liquids starts with words like “low volatility” and “non-flammable.” That makes them seem safe, but digging deeper, almost every ionic liquid has some red flags. Researchers at German universities pointed to cytotoxicity with imidazolium compounds, showing that certain variations damage fish cells and cause trouble with algae and simple organisms. They found links to DNA damage, especially involving the tetrafluoroborate part, which can split apart and release toxic products in water.
In 2010, the journal “Ecotoxicology and Environmental Safety” published a study looking at how similar compounds impact aquatic life. Fish and water fleas faced changes in reproduction, growth, and survival—not right away, but after exposure over days and weeks. Results like this say something important: just because a chemical doesn’t have a strong smell and doesn’t light up easily, it can still hurt ecosystems in the long run.
Real-World Risks
Labs take pride in controlled environments. Outside, things get messy. Ionic liquids can leave traces during manufacturing or spills, ending up in water and soil. Once there, breakdown into fluoride or boron can poison plants and build up through the food web. As someone who's spent time near refineries and chemical plants, I’ve seen how compounds considered “safe enough” behind closed doors turn out to cause headaches for local wildlife and neighbors.
There’s also the matter of what happens to people who handle these liquids on a daily basis. Most safety sheets urge gloves, goggles, and good ventilation—signs that nobody’s certain the stuff is harmless. Direct skin contact sometimes brings irritation; inhalation could cause coughing. Long-term exposure questions haven’t been answered yet, which should give pause, not comfort. There’s a pattern here, seen before with solvents and plasticizers. Only after years of use did society realize just how big their effects could be.
Better Handling and Policy
Some workable solutions exist. Always opt for closed systems and high-efficiency exhaust. People in the lab ought to use nitrile gloves and splash goggles, not because of a panic, but because risk doesn’t always announce itself up front. Waste disposal rules matter too; ionic liquids shouldn’t go down the drain. Collect, label, and make sure someone trained handles the leftovers.
Policymakers can keep pushing for longer-term studies and rules about where these substances end up. Companies designing greener chemicals have a real chance to help; less toxic, biodegradable choices can shake up the field. Until more data lands on the table, it makes sense to treat 1-hexyl-3-vinylimidazolium tetrafluoroborate with all the respect given to chemicals with unknown long-term impact.
Looking Ahead
Science rarely gives instant answers. The story with this ionic liquid argues for caution—good habits, thoughtful design, and honest conversations about what we put into our water, our soil, and ourselves. That approach saves money, saves headaches, and maybe saves lives down the line.
The Stakes of Purity in Goods We Use
Purity shapes trust, quality, and even safety in the stuff we buy—whether it’s chemicals, food, or health products. You can taste the difference with chocolate. Anyone who’s baked knows that cocoa labeled “99%” gives a deeper flavor than the normal grocery store blend. It’s not only about taste. High-purity materials—like lab reagents—can run experiments toward trustworthy results. One company’s “pharmaceutical grade” stands out because regulators, hospitals, and people like you and me count on that description meaning clean, reliable, and almost free of contamination.
Poor purity levels can bring more than off-flavors—they can put health and even business at risk. Metals or chemicals found in low-quality raw ingredients ruin machines, spoil production runs, or spark product recalls. Last year, contaminated herbal supplements led to regulatory crackdowns and steep costs for small producers. Experiences like these teach hard lessons about why purity isn’t just a buzzword. It’s a measure of whether a manufacturer values its customers enough to offer peace of mind.
Different Packaging Sizes Serve Real-Life Needs
Packaging sizes aren’t just about choice—they affect convenience, cost, and the environment. Bulk containers may look cheaper per kilogram, but sometimes that’s a false economy. A lab might order just 500 grams of powdered vitamin C to avoid waste, especially if it’s sensitive to moisture and light. In retail, a one-kilogram bag can be a good fit for families, while restaurant kitchens buy massive sacks to feed crowds.
Packaging touches on safety, too. Smaller sealed bottles can keep hands off the rest of the product. Large drums allow fast manufacturing but could increase risks if not handled right. The tamper-proof seal on a 250-gram jar isn’t just marketing—it’s the sort of feature that gives parents confidence when shopping for their families.
Bringing Transparency to the Forefront
I’ve seen how someone’s livelihood hangs in the balance when the label on a product doesn’t match what’s inside. A grower or chef who buys in bulk, only to get an inferior batch, faces tough choices—accept the loss, or pass poor quality to their customers. Recalls and regulatory action follow when standards aren’t clearly met. Transparency in labeling can help everyone along the supply chain—the scientist in the lab, the worker on the factory floor, the shopper at the market.
Manufacturers can win trust by openly sharing lab results, batch numbers, and expiration dates. Clarity about how much and what grade is in the bag or bottle helps people make smart decisions. Digital tools offer ways to scan codes and pull up purity certificates. These signals show that a company knows the stakes and respects the buyer’s right to answers.
Finding a Way Forward
Simple fixes work best. Businesses should check suppliers, test shipments, and put honest information front and center. Lazy labeling isn’t fair to anyone. Small changes like offering multiple packaging sizes and updating purity test information can push the whole market toward better practices. Regulators should expand spot checks and make it easier for whistleblowers to speak up about misleading claims. Shoppers can support brands with a track record for getting it right—not just the cheapest on the shelf.
It’s clear that purity and packaging aren’t just another line on a spec sheet. The products we choose reflect the trust we place in others, and that trust grows strongest when information is both real and accessible.
The Heartbeat of Chemistry: Dissolving and Mixing
Every time I watched sugar disappear into a cup of coffee, I learned first-hand how solubility shapes the way we approach mixtures. In the lab, this sense of transformation becomes even sharper. Solubility—the ability of one substance to dissolve in another—drives most steps in chemical reactions, drug formulation, cleaning, cooking, and industrial processes. Getting this right can mean the difference between success and wasted resources.
Water: The Universal Solvent, Mostly
Water dissolves a lot—salts, sugars, acids, bases, and many gases. Its polar nature lets it pull apart ionic and polar molecules, such as table salt (sodium chloride) and ethanol. Plenty of chemical reactions depend on how well substances mix in water. Pharmaceuticals usually use water for this reason: drugs need to dissolve before they can do their job inside the body. Some drugs, such as Aspirin, dissolve only slightly, requiring special tricks or co-solvents to work properly; others, like Vitamin C, dissolve easily.
Organic Solvents: Grease, Grime, and Chemical Craft
Not everything dissolves in water. Grease stays put on dishes no matter how hot the rinse. For those jobs, you reach for an organic solvent like ethanol, acetone, or hexane. Take nail polish remover—acetone makes short work of stubborn varnishes. In the chemical industry, selecting the right solvent means thinking about the solubility of each ingredient. A chemist making plastics or paints considers properties like "like dissolves like"—polarity matches boost solubility, while differences keep things separate.
The Importance in Environmental Science and Health
Solubility shapes environmental risks too. Highly soluble pollutants like nitrates or industrial solvents move easily through water, showing up in wells, lakes, and rivers. This movement threatens clean water supplies and public health. Many pesticides used in agriculture dissolve in groundwater, and once they're in the water, they’re hard to pull back out. Understanding these patterns is more than academic—it's about safe drinking water, healthy food, and protecting biodiversity.
Tips from Hands-on Experience
After years of mixing things for fun and work, I've seen students struggle to dissolve the wrong solid in the wrong solvent. Saving time starts with checking the polarity and structure of both the chemical and the solvent. Using a little heat usually helps, but not always—some substances break down if they get too hot. Mixing in stages or using co-solvents sometimes solves a tricky problem. In drug making, tweaking pH or adding salts can make poorly-soluble ingredients dissolve better. These solutions save money and reduce waste.
Restless Progress and Greener Choices
Using harsh solvents leaves behind toxic waste and risks for workers and the environment. That’s pushed many labs and manufacturers to look for greener alternatives. Water-based processes, bio-derived solvents, and recycled solvents have started to make an impact. Real change means both science and practical work, like safer storage, better waste handling, and regular training about risks and protection.
An Everyday Solution to an Everyday Problem
Learning the solubility properties of materials opens doors—cleaner homes, safer medicines, more efficient factories, and less pollution. Those lessons, first learned over a mug of sweetened coffee or a classroom experiment, carry through to the biggest challenges in science and industry. Knowing what will dissolve—and what won’t—saves time, resources, and sometimes, lives.