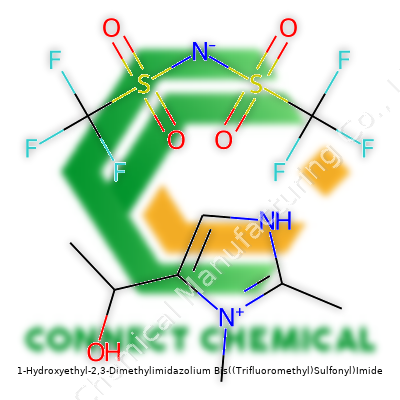
1-Hydroxyethyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: A Practical Look at a Modern Ionic Liquid
Historical Development
Ionic liquids have reshaped lab routines and industry methods in ways that carry both promise and hard lessons. Chemistry labs in the late 20th century saw the first imidazolium-based salts, but it took a slog through trial and error before researchers found actual utility outside curiosity. As scientists tuned alkyl chains and tweaked functional groups, they unlocked compounds like 1-Hydroxyethyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide. This substance didn’t roll out of nowhere—it represents decades of wrestling with flammability, viscosity, and environmental impact. Environmental scientists and process chemists both hunted for green alternatives to volatile solvents. The arrival of functionalized imidazolium salts, especially ones with the NTf2 anion, marked a shift. Properties could now be dialed in: hydrophobic, stable under heat, non-flammable. That made these substances real contenders for industrial tasks and laboratory exploration.
Product Overview
1-Hydroxyethyl-2,3-Dimethylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide, known in shorthand as [HOEtMIM][NTf2], brings together a hydroxyethyl-imidazolium cation with the bis(trifluoromethanesulfonyl)imide (NTf2) anion. What sets this compound apart centers on the hydroxyethyl handle attached to the imidazolium ring—this tweak adds both polarity and the potential for hydrogen bonding, often improving applications in catalysis or solvent extraction. The NTf2 anion brings serious chemical stability and low viscosity, two attributes sought after in demanding environments. Chemists who work with this class of material end up with fewer headaches over volatility and long-term storage.
Physical & Chemical Properties
In a busy lab or a scale-up plant, physical properties matter just as much as chemical quirks. This ionic liquid normally forms a clear, colorless-to-pale yellow viscous liquid at room temperature. It resists evaporation far better than simple organics, curbing the risk of inhalation and process losses. The melting point usually lands below –20°C, so it flows freely in cold environments. Viscosity runs a bit higher than truly simple solvents like acetone, but you still get fluid handling without the mess of solidification or sticking. Thermal stability holds up well above 200°C thanks to the robustness of the NTf2 backbone, meaning engineers don’t get stuck swapping in new fluids after heating cycles. Chemically, the hydroxyethyl group introduces a dash of amphiphilic character, pushing the boundary between completely nonpolar and polar groups, and offering up useful hydrogen bonding sites.
Technical Specifications & Labeling
Industry always checks data sheets for real-world performance: water content keeps below 0.2%, heavy metal traces below 10 ppm, and purity checks in above 98% by NMR and chromatography. Labeling reflects rigorous hazard communication. GHS symbols show acute oral and eye toxicity, while detailed instructions print right on the bottle: avoid skin contact, wear splash goggles, and store tightly sealed in inert atmosphere. Every bottle leaves the supplier batch-labeled for lot traceability, and the Safety Data Sheet usually travels right in the shipping box, giving purchasers clarity on chemical makeup and regulatory handling.
Preparation Method
Chemists follow a stepwise route in the lab. Synthesis reaches back to simple imidazole precursors, methyl iodide or dimethyl sulfate for N-alkylation, and an epoxide (like ethylene oxide) for introducing the hydroxyethyl chain. The intermediate cation salt, often with a halide like chloride or bromide as counterion, stirs together with lithium or sodium NTf2 in water or acetonitrile, then extraction into an organic phase strips out the product. Careful washing and vacuum drying prevent water from sticking around, since ions love moisture. Every stage gets NMR or mass spec confirmation to avoid byproduct confusion or costly waste.
Chemical Reactions & Modifications
In practical settings, [HOEtMIM][NTf2] adapts well to derivatization. The methyl groups resist oxidation, but the hydroxyethyl handles esterification and etherification smoothly. That’s handy for those tweaking catalytic supports or tying in chromophores for analytical chemistry. Under basic conditions, you can swap out the hydroxyl hydrogen for silyl or acyl groups. Acidic and oxidative settings rarely degrade the imidazolium system, barring strong nucleophiles or persistent radicals. The NTf2 anion, stable under nearly all solvent conditions and resistant to hydrolysis, keeps the ionic liquid around through demanding workups in synthetic or extraction protocols.
Synonyms & Product Names
No one likes confusion at the order desk. Suppliers may call this compound out as 1-(2-Hydroxyethyl)-2,3-Dimethylimidazolium NTf2, [HOEtMIM][NTf2], or its full IUPAC name, N-(2-hydroxyethyl)-1,3-dimethylimidazolium bis(trifluoromethanesulfonyl)imide. Some catalogs lump it in “hydroxyethyl imidazolium ionic liquids” or tag it by inventory numbers tied to NTf2 salts. If you dig through chemical listings, variants pop up, but that NTf2 means you’re working with a highly stable, low-flammability ionic liquid favored for modern applications.
Safety & Operational Standards
Popular as these salts have become, safety workarounds never pay off. Skin and eye irritation show up fast, especially when the cation base has hydroxy groups. NTf2-based salts can move into the air during high-heat procedures; inhalation hazards remain low, but always worth a local exhaust fan and sealed glovebox until procedures get locked down. Bioaccumulation data point to moderate persistence, and thorough wastewater treatment avoids buildup. Routine chemical handling—nitrile gloves, splash goggles, and closed transfer—cuts occupational risk. Emergency protocols mean quick washing stations when splashes or spills crop up, and all waste gets bottled for hazardous pickup, not down the sink.
Application Area
Day-to-day use stretches across several industries. In electrochemical devices, the stability under voltage swings makes this salt a pick for supercapacitors and lithium battery systems. Chemical synthesis benefits from low vapor pressure, allowing solvent extractions, supported catalyst media, and selective organic transformations that struggle in water or common organic mixtures. In separations, enhanced solubility and ionic selectivity help certain metal ions and organic dyes partition much more efficiently. Some research pushes into enzymatic catalysis, leveraging the hydroxyethyl group to stabilize delicate biological components. The low flammability and broad temperature stability open up green alternatives to volatile organic solvents, a hot topic in regulatory circles and industry trend reports.
Research & Development
R&D teams keep at this group of compounds partly because success in the lab can ripple into cleaner, safer factories. The hydroxyethyl functional group serves as a testbed for new enzyme immobilization or phase-transfer catalysis systems. Labs check for improved selectivity and reusability compared to classic ionic liquids or more toxic solvent blends. Projects testing extraction of rare earth elements or precious metals from complex ores keep showing up, since this class of ionic liquids can outperform both cost and throughput. Funding for new analytical techniques often leans on imidazolium NTf2 salts, due to their ability to dissolve metals and organics side by side without generating explosive vapors or risky breakdown products.
Toxicity Research
Toxicologists dig deeper into these compounds because regulatory green flags start with reliable animal and cell-culture data. Rats exposed to imidazolium cations with shorter alkyl chains sometimes show limited acute toxicity, but the addition of hydroxyethyl groups changes metabolism and excretion rates. Studies indicate that NTf2 salts rarely break down in environmental waters, so persistence, not acute human risk, takes the biggest concern slot. In aquatic studies, high concentrations can inhibit algal growth and invertebrate development, so containment and recovery become part of any process planning. Skin contact or inhalation at normal lab exposures runs a low risk, but uncontrolled scale-up, fires, or disposal figure into public health models. Proper chemical management policies and up-to-date training remain essential to minimize workplace exposure.
Future Prospects
Demand for robust, low-volatility solvents and electrolytes will only climb in the next decades. Projects on extracting value metals from e-waste now look at this class of ionic liquid for selective recovery without using scorchingly toxic acids. Battery and supercapacitor manufacturers see advantages in low-flammable, broadly stable electrolytes, especially in safety-conscious automotive and grid energy sectors. Process engineers aim to retrofit existing operations with less hazardous extraction and processing methods. Regulations take time to catch up to laboratory discoveries, so data collection and toxicity mapping need cooperation, not shortcuts. Chemists with boots on the ground know new technology only works if its safety and cost track with industrial need—and here, [HOEtMIM][NTf2] shows just how far ionic liquid science has come.
Stepping into the Lab: A Unique Ionic Liquid
Chemistry students and industrial scientists alike keep an eye out for materials that change the way things work. 1-Hydroxyethyl-2,3-dimethylimidazolium bis((trifluoromethyl)sulfonyl)imide stands out in the world of ionic liquids. This mouthful of a compound spent its early years tucked away in academic journals, but it has been making steady progress into real-world usage. As someone who took apart old batteries in high school just to see what made them tick, curiosity naturally draws me to chemicals that seem to have a foot in several different doors.
Green Chemistry Opens New Doors
Traditional solvents release harmful fumes and leave behind residue that builds up in the environment. Ionic liquids like this one don’t evaporate like regular solvents. That property caught the attention of labs looking for ways to run cleaner reactions, recycling their solvents with less waste. Research groups published papers showing that using this ionic liquid means fewer air emissions and less risk for explosions, especially when handling volatile or heat-sensitive substances. Data from the American Chemical Society points to a steady rise in research and patents based on ionic liquids, especially for green chemistry.
Making Batteries Better
Rechargeable batteries power cell phones, laptops, and even family cars. Engineers often struggle with flammable or corrosive electrolytes, the liquids that help move charge inside batteries. This ionic liquid resists breaking down even when heated, and barely conducts current outside the intended path, lowering the risk of battery fires. A battery research team at a large university published a study last year showing safer, longer-lasting batteries using this compound as an electrolyte. Their findings line up with a push in the energy sector to move beyond lithium-ion chemistry without trading off safety or cost.
Cleaning Up Industry
Many industrial processes chew through enormous amounts of water and acidic solvents to strip metals or separate chemicals. Those chemicals either head off to treatment plants or end up in rivers if not handled correctly. I worked in a chemical production plant over a summer, and most problems started and ended with what happened to all those solvents after a job finished. The ionic liquid under discussion here made a name for itself by replacing many of these harsh chemicals in the metal extraction process. Companies report that using it means less water is wasted and less energy is needed for cleanup.
Catalysis Finds a New Ally
Catalysts help chemicals react faster, but most require a tricky mix of metals and solvents. Experimenters found this ionic liquid provides a stable, flexible environment for catalytic reactions. That means certain pharmaceuticals or specialty plastics can be made in fewer steps, with less leftover junk at the end. Studies from European research centers show higher catalyst reusability, which saves cash and cuts down on waste.
Path Forward
None of these uses come without a few caveats. Current production costs still run high, and the long-term environmental impact of ionic liquids has not been fully mapped out. Better recycling programs and a tighter eye on toxicity help address those concerns. Government funding and private investment in green tech continue to grow, showing that interest in better, cleaner tools spreads well beyond the lab bench or boardroom.
Understanding Stability: Why It Matters
Chemicals don’t stay the same forever. Some last for years on a shelf, others break down or change in just weeks. In my time working in research labs, stability never seemed like just a technical detail; it shaped everything from budgets to safety plans. If a compound breaks down, data can turn questionable and safety slips through the cracks.
Many compounds—take simple salts or stable polymers—feel predictable. They might only react under harsh conditions. Other chemicals act more like finicky guests, threatened by light, heat, or even air. I remember a time handling a light-sensitive dye: a few extra minutes in a bright room faded my samples, forcing a restart. A stable chemical saves money, time, and sometimes even lives.
What Hurts Chemical Stability
Oxygen, light, moisture, and temperature swings ruin more chemicals than most folks realize. Some reagents, like peroxides or nitrates, take on moisture right out of the air, clumping up or even growing dangerous over time. Others, like organics with double bonds, soak up light and slowly fall apart. The change isn’t always obvious—sometimes the color looks fine, but structure silently shifts.
One lesson: never ignore the signs. If powder clumps, changes color, or smells strange, it’s not superstition—decomposition already started. Most chemical suppliers stamp bottles with recommended storage, and years of experience drive those labels. I’ve seen ignored guidelines turn a safe lab into a room with unexpected pressure build-up or leaks.
Good Storage Practices from Real Experience
Cool, dry, and dark still leads as the best advice. Refrigerators save precious enzymes and vitamins, slowing down every unwanted reaction. Freezers go further, but then condensation becomes a threat. Desiccators work well for dry storage. Even in university labs, humidity could creep in, so decent seals and regular checks made all the difference.
The right container matters. Glass reigns for many reasons—won’t pick up flavors or corrode. Amber glass beats clear glass for anything sensitive to light. Plastics vary in quality; some breathe enough to allow slow moisture seepage, so they don’t fit every job. Metal cans offer protection but can corrode with harsh chemicals. Keep fresh silica gel inside containers with water magnets, replacing them when colors change.
Label everything—not just the name, but date opened and source. In shared spaces, old unlabeled bottles linger, forgotten and mysterious. By using my own date-and-initial system, I stopped that problem early. Managing stocks smartly and rotating supplies cuts down on expensive waste and risky surprises.
Fixing Instability Issues
No one can freeze time, but a few tactics help. Break bulk chemicals into smaller bottles to avoid repeated opening. For ultra-sensitive materials, try storing under inert gas like argon. If degradation plagues your workflow, switching suppliers or even compounds sometimes makes more sense than endless tweaks. Check with experts—manufacturers and safety officers offer advice honed from years of hands-on mishaps and fixes.
Pursuing Safe, Reliable Use
Every project should start with honest research on storage needs. A little up-front caution prevents headaches, injuries, and expensive reruns. It’s not about paranoia, just smart habits drawn from a lot of stained lab coats and trial runs. In the end, proper storage keeps science trustworthy, safe, and productive for everyone involved.
Running Into Unknowns in the Lab
Every chemist and lab tech finds themselves wondering about the stuff they use daily. I remember walking into the storeroom as a rookie chemist, seeing bottles of acetone, methanol, and dimethyl sulfoxide, and feeling uneasy about mixing them with random plastics or tubing. Stories float around about ruined pumps or embrittled tubes after a single careless experiment. Solvent and material compatibility isn't just a technical detail—it's a matter of getting the job done safely and avoiding expensive mistakes.
Everyday Solvents and Their Odd Relationship with Lab Materials
Take acetone, for example. Handy for cleaning glassware, dissolving organics, and sometimes degreasing. Acetone chews through polystyrene and many other plastics in what feels like seconds. I've watched a polystyrene petri dish turn into a warped mess right in my hands. I started paying more attention to material data sheets and compatibility charts. Polyethylene and polypropylene stand up to acetone and a handful of tough solvents. On the other hand, PVC and polycarbonate struggle to withstand many common solvents over time, even if failures aren't immediate.
Glassware is the old reliable. Borosilicate glass takes on just about anything outside of hydrofluoric acid or a blast of strong alkali. Laboratories lean on glass not just for tradition, but because generations have proven it works. Stainless steel also handles a wide range of solvents, but strong acids and halides push its limits.
Chemical Mishaps and Their Costs
Ignoring compatibility hits more than just the budget. Leaks, warping, and oozing bottles spill dangerous material right onto lab benches or into waste disposal systems. Sometimes, solvents leaching into tubing throw off analytical results. Once, I traced weird GC/MS peaks back to a plasticizer that washed out from the tubing—an easy miss until you dig deep. Labs that don't respect compatibility pay for it with unreliable results, higher repair bills, and sometimes hazardous exposure.
Why Compatibility Testing Helps Everyone
Manufacturers usually list which plastics or rubbers stand up to which chemicals, but those charts skip some real-world details. In practice, mechanical stress, sunlight, and repeated cleaning all shorten the lifespan of even the toughest materials. My colleagues and I started doing simple soaking tests before switching to different tubing or bottles. Placing a length of unknown tubing in a solvent overnight tells you much more than a generic chart.
The same goes for seals and gaskets in instruments. Nitrile O-rings work for many aqueous solutions, but they fail quickly in chlorinated solvents. Viton or PTFE components last longer under a wider range of solvents. Labs that stock these can switch between experiments faster, saving downtime.
How Labs Can Avoid Nasty Surprises
Training makes all the difference. Sharing stories—both successes and disasters—turns lists of solvent compatibilities into lessons you remember. Digital tools make this research easier. Lab supply companies provide online compatibility checkers, and a lot of labs keep laminated printouts stuck to the chemical storage cabinets.
Investing in the right materials up front saves trouble. It’s tempting to buy the cheaper tubing or storage bottles, but the savings disappear if a batch of product spoils or an instrument fails. I’ve learned to check before I try new combinations, even with something as simple as a different pipette tip. With rising costs for everything from reagents to repairs, it makes sense to get compatibility right the first time.
Product Safety Means Knowing the Basics Cold
Any workplace using chemicals or specialized materials stands just one mistake away from an accident. People sometimes roll their eyes at safety briefings, but routines exist for a reason. Placing the right instructions in the hands of workers keeps everyone healthier in the long run. I’ve seen seasoned crew members ignore a simple glove rule and wind up with burns that could’ve been avoided. No one wants downtime, doctor bills, or an incident on their record.
Straightforward handling procedures do more than tick a compliance box. They set a clear line between careful work and risky shortcuts. The best protocols boil down potential hazards, list protective equipment, and explain the cleanup steps in case of a spill. For example, hydrofluoric acid will send someone to the ER if it touches bare skin. That fact alone justifies double-checking gloves and face shields. When protocols lay everything out, workers start building good habits instead of guessing their way around the worst-case scenario.
Supporting Employees with Reliable Data
Good safety data pulls its weight on the shop floor. Labels need to highlight the physical risks—fire, explosions, toxicity. Yet, the data sheet does more than warn people. It answers questions about safe storage, ventilation, and first aid. Sometimes folks overlook the details like proper air canisters, but a chemical vapor in a poorly vented space leaves everyone breathing trouble. Real-world examples speak loudest: I once knew a welder who trusted an old storage drum, only to discover it held a volatile solvent. That story made “double-check your labels” a catchphrase at that job site.
Peer-reviewed information builds trust. Just because something carries a fancy brand logo doesn’t mean it’s harmless. Data from recognized experts or agencies—think OSHA or NIOSH—sets a high bar. These sources balance technical detail with practical advice. For instance, they lay out how long a person can wear a respirator before it needs swapping, or list the exact soap for a decontamination incident. No sense playing fast and loose with that kind of know-how.
Valuing Experience, Not Just Checklists
Veteran workers know procedures by heart, but even pros run into new variants or suppliers. Keeping up-to-date documents at every workstation saves time and prevents confusion. Basic reminders—like washing hands before eating or not storing food near chemicals—stop many health headaches before they start. Safety isn’t just about the rare, headline-grabbing disaster; it’s about stopping small slipups that grow into big problems.
I’ve heard some folks bash “paperwork” as pointless. Experience speaks loudest: the person with the most memorable safety story is never the one who skipped steps and got away with it. It’s the individual who caught a problem early, spoke up, and kept their crew out of harm’s way. These experiences show how ordinary people shape a culture of looking out for each other—and that’s what fixes small mistakes before they snowball.
Fixing Weak Spots in the System
No system gets it perfect every time. Clear labels fade. Training sessions get skipped. People get comfortable and cut corners. Some fixes don’t need a budget—just a renewed sense of shared responsibility. Asking employees to walk through a procedure together when bringing on a new product beats e-learning modules every time. Rotating storage locations for flammable or reactive substances can also make workers think twice before grabbing the wrong drum. Small changes, like updating worn-out labels or adding extra gloves at a mixing bench, save more trouble than they cost.
The safest workplaces don’t use up lucky breaks. They run on respect: respect for rules, respect for the data, and above all, respect for each other’s health.
Why Material Choice Shapes Battery Futures
Choosing the right compound for batteries shapes everything from our smartphones to the future of transportation. Years working with battery testing showed me that not every promising chemical on paper survives the heat, stress, and endless cycles that real-life batteries demand. A material’s promise hinges on more than conductivity or capacity numbers on a spec sheet. You notice what really matters only after dozens of charge-discharge cycles — stability, safety, and compatibility with existing components.
Evaluating Performance in the Real World
A big claim about a breakthrough compound often lights up headlines. Lab trials generate impressive results, but full-scale batteries tempt unpredictability. Certain compounds degrade or form dendrites — tiny metal filaments that short-circuit batteries and risk fires. Take lithium metal, once hailed as a silver bullet but commonly struggling with stability issues. Compounds showing robust performance over hundreds of cycles, without sharp drops in capacity, attract real interest. Think of lithium iron phosphate’s rise by delivering steady power and a strong safety record, despite not matching every number of pricier competitors.
Key Properties to Look For
Chemists watch for high electrical conductivity paired with strong thermal stability. If a compound breaks down above typical battery temperatures, designers walk away. I remember testing a polymer electrolyte that promised high ion flow, but it warped and lost shape when things heated up. In many labs, the scene repeats: what works at 30°C turns unreliable by 60°C. Compounds resisting corrosion, keeping stable under voltage, and handling high current loads become real contenders.
Cost, Scalability, and Sustainability
Price tags often block even the most advanced material from wide use. Cobalt, for example, pushed battery prices up for years and drew backlash for ethical mining concerns. Manufacturers look for compounds sourced abundantly, with simple, low-energy synthesis methods. A solution could lie in new processing methods, or switching to materials like sodium or sulfur with lower supply-chain risks. I’ve seen start-ups pivot fast after raw material prices spiked, sometimes pushing forward greener and more affordable chemistry.
Overcoming Challenges and Looking Ahead
Novel compounds face tough barriers outside the lab. Electrolytes that seem promising sometimes react with electrodes over time, slowly eroding performance. Improving interfaces with coatings, or racing to develop solid-state configurations, helps mitigate these issues. Partnerships between universities and manufacturers speed up this learning — real-world testing catches a thousand little problems before products hit the shelves.
In my time with battery integration projects, collaboration fueled progress. Bringing together chemists, engineers, and supply folks prevented costly delays and dead-ends. Teams caught issues like unexpected swelling or voltage drift early, finding creative fixes that made a difference on the factory floor.
The Road to a Better Battery
New compounds generate excitement and investment, but patience counts. Rushed promises end up in recycled press releases or recalls. Real progress comes from incremental improvement, thorough real-world testing, and an honest look at safety, price, and availability. Every material that moves us closer to safer, longer-lasting, and more sustainable power changes what’s possible for everyone — on the road, in hospitals, and inside homes. Those of us who’ve spent late hours in the lab know: the next breakthrough could be a test away or require years more grit.