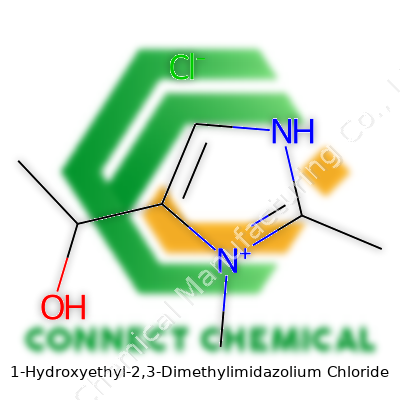
1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride: A Deep Dive
Historical Development
Throughout the past few decades, chemists have chased new ionic liquids with characteristics that break the mold. When 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride entered the scene, researchers took note because it didn’t just tick off boxes on a checklist—it changed expectations about what ionic liquids could do. For years, the imidazolium family has shaped green chemistry, but this particular compound—a mouthful at first glance—emerged from this crowd through targeted synthesis in labs focused on sustainability and energy efficiency. Its evolution leans heavily on refining classic alkylation techniques and adopting environmentally responsible feedstocks. Witnessing lab notes or journals from earlier syntheses, trial-and-error ruled, with incremental discoveries driving the confidence that this ionic liquid would matter in both academic and industrial settings.
Product Overview
1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride is part of the ever-expanding catalog of ionic liquids marked by the imidazolium ring and specialized alkyl groups. The hydroxyethyl side chain gives this compound a polarity edge, nudging it into unique solvent territory. Unlike standard organic solvents that rely on volatile and sometimes hazardous bases, this substance offers a blend of solvating power and thermal stability. Labs prize it for its ability to dissolve everything from cellulosic materials to transition metal salts, extending its application field far beyond academic chemistry. Each bottle arrives in labs with high purity, a solid crystalline form under ordinary conditions, and a comfortingly clear labeling that tells researchers exactly what to expect.
Physical & Chemical Properties
Pouring 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride onto the balance, I notice its hygroscopic nature—it attracts moisture quickly and clumps unless tightly sealed. Melting occurs at moderate temperatures, something like 90-120°C depending on sample purity, so room temperature storage usually keeps it solid. Its ionic character brings low volatility, with minimal odor compared to traditional volatile organic solvents. I’ve seen it blend seamlessly with water and many alcohols, but it resists dissolving in most nonpolar solvents. Chemically, its imidazolium ring guards against harsh bases and oxidation, so degradation during handling proves rare. In practice, its carefully tuned structure not only provides robust solvation and ionic conductivity, it also cuts risk of decomposition in both routine and stress conditions.
Technical Specifications & Labeling
Each container lists specifications like molecular weight, purity, potential heavy metal content, and water percentage, often supported by a batch analysis document. Chemists and regulatory professionals both benefit from unambiguous CAS numbers, precise labeling of storage temperature limits, and recommendations for air-tight, moisture-free storage. My own experience in quality control flagged products that skimped on these details—misunderstanding a purity grade or missing out on water content can torpedo a sensitive experiment or production batch. Packaging favors dark glass or high-density polyethylene, protecting from unnecessary light and accidental contamination.
Preparation Method
Lab-scale synthesis of 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride usually starts with straightforward alkylation of 2,3-dimethylimidazole. A 2-chloroethanol reactant adds the hydroxyethyl group under controlled heating and solvent conditions, forming the cationic structure. Subsequent ion exchange or direct reaction with hydrochloric acid sets the chloride as anion. I’ve watched skilled technicians maintain tight temperature and pH windows, since small shifts can nudge the mixture toward unwanted side-products. Afterwards, repeated extraction and recrystallization drive up yield and purity, separating the desired product from unreacted reagents and byproducts. Scale-up for industry uses higher-efficiency reactors, sometimes continuous setups, which push volumes while keeping the same strict controls on reaction kinetics and impurity levels.
Chemical Reactions & Modifications
This ionic liquid’s structure not only makes it stable—it simultaneously allows chemical modifications. Reactions target the hydroxyethyl group for esterification, etherification, or even incorporation of reported functional groups. Labs regularly exploit this to tailor solvents for catalytic processes or custom extractions. In my own work, attempts to bond rare-earth chelators to the hydroxy position yielded new extraction agents that outperformed generic ionic liquids by orders of magnitude. The imidazolium ring can also see substitution for advanced applications, influencing hydrophobicity or bringing task-specific properties. Each modification shifts physical parameters—the melting point rises, viscosity drops, or solubility increases—expanding the utility of the original chloride salt.
Synonyms & Product Names
The world of chemicals rarely sticks to just one name. 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride appears on invoices, chemical catalogues, and research papers under several aliases. I’ve seen phonetic simplifications, like “HEdmim-Cl” or “C2OH-MIM-Cl”. Regional manufacturers prefer slightly altered nomenclature based on IUPAC or local standards. Some suppliers attach proprietary trade names, particularly when their product supports a patented formulation or application. Strict record-keeping in my own workflows left me double-checking these synonym lists before ordering, preventing mix-ups between subtly different substances.
Safety & Operational Standards
No chemical with innovative potential gets a pass on safety. Handling 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride needs attention to personal protective equipment—gloves, goggles, and solid ventilation. It avoids dramatic hazards like flammability or inhalation toxicity, but direct skin contact can cause irritation and prolonged exposure brings risk in sensitive individuals. Emergency protocols in my lab flagged quick access to eyewash stations and spill containment, especially considering this compound’s hygroscopic pull, which turns spills into slippery patches. Safe waste disposal matches ionic liquids in general: dissolved or diluted product needs collection in pre-labeled containers bound for controlled chemical destruction rather than regular drains or trash. Audits in facilities using this substance highlight routine refresher courses on handling procedures and emergency responses.
Application Area
This compound leaves its mark across more fields than a lot of specialty chemicals. Green chemistry circles praise it for dissolving cellulose, making biofuel production and biomass processing more efficient than legacy solvents. Electrochemists appreciate its ionic conductivity for new-generation batteries and supercapacitors, where traditional electrolytes either degrade or lack the right thermal window. In catalysis, it serves as both solvent and co-catalyst, supporting clean transformations not possible with standard alternatives. My own exposure goes back to attempts at extracting critical metals from electronic waste, with this ionic liquid delivering higher selectivity and recovery rates than other candidates. Industry partners keep tabs on its promise in pharmaceutical synthesis, carbon capture, and specialty separations.
Research & Development
Research groups and corporate labs maintain a steady focus on extending the knowledge and utility of 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride. Teams take up projects on finding alternative, even greener, synthesis pathways—often swapping fossil-derived reagents for bio-based ones. Computational chemists map the influence of minor structural tweaks on physical properties, hoping to chart the path for designer solvents. I’ve seen collaborative efforts bring together chemical engineers, environmental scientists, and industry to match this compound with emerging challenges—battery recyclability, waste minimization, and improving energy efficiencies. Peer-reviewed papers keep reporting advances in polymer solubilization, rare-earth extractions, and catalytic cycles relying on its unique solvation.
Toxicity Research
Questions on environmental and health safety never fade into the background. Recent toxicity studies highlight a generally lower acute toxicity profile compared to many conventional solvents, but chronic effects receive close scrutiny. Aquatic toxicity screens raise concerns about bioaccumulation, especially as ionic liquids make their way from laboratories to manufacturing scales. In animal studies, scientists found low-to-moderate toxicity when compared with imidazolium salts containing longer alkyl chains. But each new batch we synthesize brings the need for batch-specific verification. Researchers dig deeper into breakdown products and persistence, with the consensus pushing for better data before advocating for broad, unchecked use. Lab environments today err on the side of caution, integrating the latest findings into routine risk assessments and disposal methods.
Future Prospects
Looking ahead, 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride stands at the edge of mainstream adoption for advanced materials processing, energy devices, and specialty separations. Expectations keep building as battery and biofuel sectors look for more sustainable chemistries with a lower environmental footprint. Ongoing pushes for full life-cycle analysis and green chemistry audits keep researchers honest, making every claim of reduced toxicity and superior efficiency subject to rigorous testing. I see a wave of new start-ups and established players both racing to patent improved derivatives, scalable recycling processes, and more benign breakdown pathways. High-throughput computational chemistry and automated screening promise to shrink the time from discovery to application, with this ionic liquid’s framework at the center of those developments. If my own experience holds true across the field, its value will come from adaptability, a proven record in key applications, and a willingness by both public and private sponsors to invest in long-term environmental risk management, not just immediate process gains.
Why This Chemical Gets Attention
People who walk into a chemical plant might hear names like 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride and wonder whether someone made a typo. The reality is that this chemical plays an important role in shaping the way certain industries handle challenging processes. In my years writing about research breakthroughs and new patents, I have seen how these chemicals often do some of the heavy lifting in applications you wouldn't expect, especially in “green” chemistry moves that seek to protect both human health and the environment.
What Does It Actually Do?
1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride stands out as an ionic liquid—a category of compounds known for replacing volatile organic solvents. Traditional solvents contribute to air pollution and, frankly, cause headaches both figuratively and literally. Companies working on biomass conversion—turning wood, straw, or waste into biofuels and biochemicals—have started turning toward chemicals like this one. Scientific publications from reputable universities describe how they use it to dissolve cellulose, the tough plant material that makes up much of a tree or a corncob. Without clever solvents, anyone trying to break down cellulose for greener energy runs into obstacles.
I sat in a lab once where two researchers, frustrated with older solvents, tested this compound on wheat straw. Instead of reeking fumes and flammable hazards, they saw stronger performance, a dramatic drop in waste, and better yields of useful sugars. The stuff seemed to fit the new world of focused, sustainable chemistry. Industry leaders like BASF and academic experts have pointed out that these ionic liquids can help reduce total hazardous emissions. One study out of Germany showed a 40% decrease in greenhouse gas emissions in pilot applications compared to standard solvents.
What’s At Stake?
Cleaner energy production should matter to just about everyone who has seen the toll of smoggy cities or heard farmers worry about eroding land. Chemicals like 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride let companies do the tricky work of converting natural fibers into fuels with fewer side effects than before. The downside? Cost. This compound costs more per kilo than plain ethanol or acetone, which means it rarely gets used outside of specialized labs or new facilities. If we want to see it at scale, costs must drop or governments must reward companies for cutting pollution.
Safety is the next thing on the list. The science looks promising, but regulators do not fast-track approvals for new chemicals, especially those with potential toxicity risks if spilled or misused. A few years back, I heard from safety officers dealing with ionic liquid spills who pointed out the need for better data and cleanup protocols.
Looking for Solutions
Sometimes industry needs a nudge. Governments could consider tax credits for facilities that swap out old solvents for greener ones. Universities can keep testing breakdown rates and ways to recover and recycle these chemicals after use. Chemical companies benefit from collaboration—sharing new findings with each other to drive down price and up the safety.
1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride might sound like just another item in a catalog. It actually signals progress toward safer, more sustainable production. With the right partnerships, it could shape the path to cleaner energy and materials, without trading away safety or cost-effectiveness.
Why Storage Matters
1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride, a mouthful to say but not uncommon in research labs, sits on shelves where safety slips through the cracks. Many people keep their chemicals with little thought beyond fitting them in a cabinet or drawer. Yet, with ionic liquids like this one, poor storage can lead to problems ranging from lost potency to ruined experiments or even safety risks. From my own days helping in a university lab, I remember what happened when we ignored proper storage guidelines—unmarked containers, ruined reagents, and headaches for everyone down the line.
Key Storage Conditions
Dry EnvironmentThis compound draws moisture from the air easily. Leave a jar open, and crystals slowly turn sticky. Moisture can even alter the properties researchers depend on, sometimes changing how the compound reacts. Keeping it dry starts with good habits: always close containers tightly, and tuck them away in cabinets with desiccant packs. In my experience, investing in a lab-grade desiccator pays off quickly. Silica gel packets swapped out every month or so keep things fresh and dry.
Keep Out of the SunDirect sunlight increases temperature swings and can kickstart chemical changes that most labs want to avoid. I have seen sealed vessels sweat and react simply by sitting near a sunny window. Store bottles in shaded cupboards, or better yet, dedicated chemical storage cabinets with opaque doors.
Control the TemperatureMany workspaces bounce between freezing air conditioning and the sweat of summer afternoons. Ionic liquids like this one handle fluctuations poorly. A chemical fridge or designated storage at a steady, moderate room temperature (20-25°C) keeps the compound stable and prevents unwanted reactions that heat or cold might spark. I’ve always found a temperature log posted on the storage unit helpful, both to remind everyone of best practices and to track any power outages.
Sealable, Properly Labeled ContainersFor years, old coffee jars and scratched labels were the norm in small labs I visited. That led to confusion and near misses. Good storage starts with high-density polyethylene or borosilicate glass bottles with fresh labels. Clear labels listing content, date received, and possible hazards prevent mistakes—one glance should tell a new staff member what’s inside.
Segregate Incompatible MaterialsEven if this imidazolium salt doesn’t react with common acids or oxidants, don’t gamble. Keep it on shelves that don’t share space with strong bases, acids, or flammable reagents. Separate shelving cuts risk, and it’s standard in industry and academia alike. Chemical compatibility charts stuck to cupboard doors help avoid accidental mixing.
Potential Hazards and Solutions
Incorrect storage increases exposure risks. Inhaling or touching small spills from this salt usually brings little harm, but allergies and skin irritation aren’t rare. Safety Data Sheets detail risks, but the best protection always starts with gloves, goggles, and common sense. Leaky caps or cracked jars create messes in shared spaces, and if the compound absorbs too much water, it can become unusable, costing real money over the long run.
Good storage relies on three habits: regular checks for leaks, keeping the environment controlled, and clear labeling. These steps keep the chemical viable, protect the people handling it, and show respect for the resources invested in research or production.
Why Structure Matters in Chemistry
People glance at a chemical formula and often move on, but every angle and bond in that drawing is a clue. In the world of molecules, structure shapes everything—how a drug relieves pain, how a cleaner breaks down grease, or how a polymer insulates your house. The difference between a life-saving medicine and a mild irritant might come down to the rearrangement of a couple atoms.
How Chemists Map Out a Structure
Drawing the chemical structure of a compound means more than copying lines on paper. Chemists look at each atom’s placement, every single bond, ring, or branch along the skeleton. Names like “hydroxyl” or “carboxyl” tell you something about how the compound will act in your bloodstream, or inside the soil. X-ray crystallography and NMR scans have changed this work—once, scientists needed to break apart a mystery molecule to figure out its pieces. Now, machines let us peek at the shapes directly, piecing together 3D structures from slices in a way that reminds me of solving a complicated jigsaw puzzle.
Molecular Weight: More Than Just a Number
Molecular weight, measured in Daltons or unified atomic mass units (u), holds real-world significance. Many routine tasks in the laboratory—mixing a solution, measuring out a medication dose—rely on this number. If you mess up the calculation, your results can miss the mark or send someone straight from pharmacy to hospital. A practical lesson: One time, I calculated the molecular weight of ascorbic acid (Vitamin C) wrong in a college lab. The solution ended up far too weak to react, proving how paying attention to every hydrogen and carbon atom translates into real, sometimes costly, mistakes.
Tools to Make Life Easier for Chemists
Today, online databases like PubChem, ChemSpider, and the Protein Data Bank provide instant access to chemical structures and their weights. You type in a name or a smiley-string, and detailed structures pop up in seconds. These resources cover tens of millions of compounds and continue to grow as researchers add new discoveries. Before the internet, stacked handbooks and lots of squinting went into tracking down structures. Now, even a high school student can pull up a 3D model of caffeine or dopamine without hunting through paper files.
Accuracy and Honesty in Reporting
I’ve seen people cut corners, pulling weights from unverified websites or relying on out-of-date versions of popular handbooks. This can go wrong, fast. Reliable science depends on scrupulous data collection—double-checking sources like peer-reviewed journals and asking colleagues for a second opinion. In clinical labs, incorrect structure or mass calculations can spell disaster for entire experiments or patient safety.
Room for Improvement
Even now, mistakes creep into public databases or popular textbooks. Routine audits by teams with expertise make a huge difference, but crowdsourcing and open peer review could spot errors sooner. Educators can push students toward trusted databases, teaching good research habits from the ground up. Pursuing accuracy upholds public trust in science and keeps dangerous errors out of labs and industries.
Understanding the Chemical
Take a look at the growing list of odd-sounding chemicals popping up in clean energy labs, and you’ll soon stumble on names like 1-hydroxyethyl-2,3-dimethylimidazolium chloride. It’s a mouthful, but it comes up for good reason. Researchers use this ionic liquid as a solvent and catalyst thanks to its special blend of physical and chemical properties. It doesn't evaporate easily, handles heat well, and can dissolve plenty of tricky materials. That’s why it’s common in green chemistry and battery research.
Looking at Toxicity and Hazards
Whenever a lab introduces a new chemical, folks who work there ask, “Is this safe to handle?” The Material Safety Data Sheets (MSDS) for most imidazolium-based ionic liquids like this one often say “limited data.” That’s a red flag for anyone used to handling chemicals. Some imidazolium salts show signs of toxicity in aquatic life, and a few studies suggest that they can mess with cell membranes in both fish and mammals.
Breathing in dust or having skin contact may cause irritation—even burns if left long enough. One time, while doing hands-on chemistry work with similar ionic liquids, a small spill on my wrist led to a stinging rash that lingered for days. Some forms can penetrate gloves faster than you’d think. That tells me that just because a chemical isn’t classified as a “traditional solvent” doesn’t mean it should be handled casually.
Assessing Exposure Risks—Factoring in Usage
Labs rarely deal with giant vats of this stuff. Instead, it’s usually a beaker or two—maybe less in pilot projects. But scale matters. Industrial users might store gallons, sparking bigger spill and waste risk. Tests on similar chemicals show persistence in soil and water, with potential for low-level contamination over time. European chemicals regulators like ECHA have worked on classifying imidazolium compounds, and several already carry “hazardous to aquatic environment” warnings. Their toxicity depends on side groups on the molecule, but the imidazolium core often ends up flagged as a flammable, irritant, or aquatic toxicant.
There isn’t proof of cancer risk from this specific compound, but gaps in research keep many cautious. Current guidance suggests avoiding skin and eye contact, using solid protective gear, and keeping spills away from drains. These small steps make a big difference. They echo what my old organic chemistry mentor used to say: “Don’t take shortcuts on unknown chemicals. You could pay the price years later.”
Solutions and Safer Practices
A big part of chemical safety is about substitution and minimization. If something less hazardous gets the job done, try to pick it instead. For 1-hydroxyethyl-2,3-dimethylimidazolium chloride, using small amounts in closed containers, with spill trays and fume hoods, cuts risk. Staff training and easy access to eye wash stations add extra layers of protection. Anyone storing it for industrial use can set up wastewater capture, secondary containment, and strong air handling.
Good hazard labeling, up-to-date MSDS printouts, and regular risk reviews go further than posters warning about “toxicity.” Chemical stewardship comes down to personal responsibility and workplace culture. It’s not about fear-mongering, but honesty about what’s known and admitting what remains murky.
Moving Forward with Care
Bottom line: Until long-term studies catch up, it’s better to treat 1-hydroxyethyl-2,3-dimethylimidazolium chloride with the same respect as any other under-studied chemical. Take small-scale exposure seriously. Push for greener alternatives. Remember that safe handling is less about paperwork and more about day-to-day habits learned from people who have stayed injury-free over long careers.
Understanding the Stakes
Lab workers and plant technicians spend a lot of time around chemicals that hold promise for industry but demand real care. 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride pops up in a variety of synthesis and green chemistry projects. Some see only its advantages—high solubility, ionic nature, stability. Yet, working with it teaches respect for how quickly a spill, poor ventilation, or casual disposal practice can turn convenience into risk.
Personal Protective Measures Matter
A pair of goggles and gloves may seem like an obvious precaution to chemists, but it goes beyond that. This compound can irritate skin and eyes. I remember the sting of a careless splash, even through a sleeve. Proper gloves, not just latex but nitrile-rated ones, stop that burn. Safety glasses block the fine droplets. Suits or lab coats—fully buttoned, no rolled sleeves—carve out a barrier that limits accidental skin exposure.
Nobody thinks a fume hood is special until the day a bottle opens up and the smell slaps you in the face. The best labs keep local exhaust running, swapping contaminated air before folks get exposed. Breathing masks hold value on high-activity days or when working in older spaces with questionable airflow.
Spills Happen, Responses Define Safety Culture
Nobody predicts every spill. That’s just the way of chemistry. The response, though, shows how much a group values safety. Scattering absorbent material—no paper towels, but proper chemical spill kits with neutralizers and pads—keeps the spread contained. Sweeping up, sealing waste in labeled containers, and logging the incident teaches others not to repeat mistakes.
Once, I watched a newer lab member panic and try to wipe up a spill with a lab rag. Training turned that moment around—showing them where to find the spill kit and how to avoid skin contact. Every new recruit needs hands-on practice before they step near open containers.
Storage: Containment Over Convenience
Too many accidents trace back to mismatched storage. 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride deserves its own spot away from acids, oxidizers, or open sun. Chemical-grade sealed bottles, even doubled up in polyethylene bags, prevent moisture from getting in and vapor from getting out. Labels, legible and durable, reduce error—nobody should need to guess what's in the jar when seconds count.
Waste Disposal Calls for Accountability
Sending chemicals down the drain might seem tempting at the end of a long shift. Environmental fate studies show that chemicals like this cationic liquid hang around and impact local ecosystems. Local laws set the rules, and they tend to be tough for a reason. Hazardous waste managers expect separation of ionic liquids from common organic and inorganic trash. Sealed drums, paperwork, and registered carriers form the backbone of responsible disposal.
Those who work with regulatory bodies find it helps to keep meticulous logs: volume, nature of the waste, destination, and date transferred. It’s more than paperwork. It means the facility treats both people and the planet as priorities. Chemical recycling may play a growing role, especially as green chemistry gains ground and practices shift toward less harmful alternatives.
Training and Vigilance Set the Standard
No manual captures every scenario. Continuous improvement comes from experienced workers sharing what works and where corners invite problems. Routine drills, review sessions, and honest debriefs about near-misses all create a workplace where safety isn’t extra—it’s built in.
A community of practice—built on sharing mistakes, highlighting practical fixes, and keeping up with advances in safety science—turns a hazardous material into a managed risk. That’s both the moral and practical duty for anyone handling 1-Hydroxyethyl-2,3-Dimethylimidazolium Chloride.