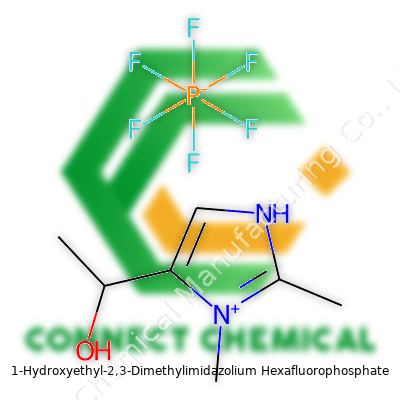
1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate: From Laboratory Curiosity to Modern Application
Historical Development
Chemists started looking for alternatives to volatile organic solvents as far back as the 1990s. The need for safer, more stable, and eco-friendly options led to experiments with ionic liquids. At that time, many researchers faced challenges such as limited choice and poor thermal stability. Focus shifted to imidazolium-based salts, and 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate emerged as a reliable candidate. This compound’s balanced structure struck a chord. It attracted interest not just for its unique salt-solvent duality, but also for performance across different environments. Over time, improvements in synthesis refined both purity and yield. Reports from leading journals show how industry adoption began initially with specialty laboratories and gradually became mainstream in academic and industrial research settings.
Product Overview
1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate is an ionic liquid, presenting itself as a colorless to pale yellow viscous liquid under ambient conditions. This product falls within the category of room temperature ionic liquids — compounds valued for low vapor pressure, favorable conductivity, and thermal stability. Unlike conventional organic solvents, it resists evaporation, cuts down on emissions, and limits worker exposure risks. Laboratories order it directly for use in organic synthesis, electrodeposition, and catalysis. Purity grades typically exceed 99%, helping researchers avoid unwanted impurities in precise experiments. Bulk packaging matches industrial scale-up, while small vials offer flexibility to university or small business labs.
Physical & Chemical Properties
1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate displays a melting point below room temperature, ensuring liquid state across most climates. The compound is highly polar, which proves useful for dissolving materials that resist traditional solvents. Its density ranges between 1.2 to 1.4 g/cm³, and high ionic conductivity makes it popular for applications in electrochemistry. Being moisture sensitive, it absorbs atmospheric water, which can shift its properties in open air. The hexafluorophosphate anion imparts thermal stability up to roughly 180°C, and chemical compatibility supports reactions with a range of organic and inorganic compounds. The unique hydroxyethyl group improves miscibility with polar substances, creating new possibilities in extraction and separation science.
Technical Specifications & Labeling
Reliable suppliers measure and display product specifications clearly on every container. These usually focus on molecular formula (C8H16F6N2OP), CAS number, appearance, purity (determined by HPLC or NMR), moisture content, and shelf life. Shipping materials stress secure handling, as the hexafluorophosphate content brings particular regulatory attention. Each label features hazard pictograms and warning phrases compliant with global (GHS) and regional rules, such as the European REACH regulation. Certificates of Analysis (COA) accompany shipments for traceability. Lot number tracking connects each bottle to its synthetic batch, providing accountability across international borders.
Preparation Method
Synthesis of 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate commonly starts with 1-methylimidazole. Introduction of a methyl group at the 3-position occurs via methylation, often using methyl iodide under strictly controlled conditions. The hydroxyethyl chain is added by allowing the dimethylimidazole core to react with an epoxide, such as ethylene oxide, resulting in the N1-hydroxyethyl derivative. Quaternization is closely monitored to prevent byproducts. The final step is anion metathesis, exchanging the hydrochloride or iodide anion for hexafluorophosphate by treating with potassium hexafluorophosphate in aqueous solution. Filtration and vacuum drying remove residual salts, and further purification by column chromatography or recrystallization can yield laboratory-grade product.
Chemical Reactions & Modifications
This ionic liquid supports a broad variety of chemical reactions. Electrochemical reduction and oxidation benefit from enhanced conductivity and unique solvation effects. It serves as a solvent or co-catalyst in nucleophilic substitutions, Diels-Alder reactions, and transition metal-catalyzed couplings. The hydroxyethyl function allows additional modifications — it can undergo esterification, etherification, or be functionalized further for targeted tasks such as ionic liquid-supported catalysts. The cation remains stable under mild acid or base, while strong nucleophiles or oxidative stress can decompose the hexafluorophosphate anion, so process conditions matter. In recycling efforts, phase separation or distillation retrieves the bulk ionic liquid for reuse, supporting green chemistry practices.
Synonyms & Product Names
Chemists may encounter alternate names such as 1-(2-Hydroxyethyl)-2,3-dimethylimidazolium hexafluorophosphate, HE-DMIM PF6, or simply the abbreviation HEDMIM PF6. Each name reflects the substance’s structural features but the core product remains the same. Large suppliers and chemical catalogs often segment product lines by cation and anion, making ordering straightforward for repeat customers in research or pilot plants.
Safety & Operational Standards
Safe operation around 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate relies on respect for strong oxidizing potential and the PF6- anion. While it has lower volatility than solvents like chloroform, workers need gloves and eye protection, especially in poorly ventilated areas. Direct skin or eye contact can irritate. Hexafluorophosphate salts generate hydrofluoric acid upon exposure to strong acid or fire, an acute hazard that demands calcium gluconate gel and medical readiness if accidental exposure occurs. Proper storage away from moisture and acids extends shelf life, and spill kits with fluoride neutralizers should stay on hand. Regulatory filings in Europe and North America require compliance with REACH or TSCA for bulk shipping. Labs and process plants keep a focus on worker education to prevent new staff from missing important safety details.
Application Area
Researchers and industry professionals find value in this ionic liquid across many specialties. It replaced traditional solvents in advanced organic synthesis, improving selectivity and cutting emissions. Electrochemical cells use it for batteries, capacitors, and sensors, where the ionic medium supports high conductivity and voltage stability. Analytical labs appreciate its tunable polarity for chromatographic separations and sample extractions. Catalysis researchers use the hydroxyethyl group to anchor metal complexes, tuning the liquid for chemical transformations without requiring hazardous solvents. Pilot-scale chemical plants explore its role in biomass processing and pharmaceutical intermediates. Some academic programs promote ionic liquids as a teaching aid for green chemistry coursework.
Research & Development
Constant research improves the performance and affordability of 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate. Publications over the past ten years focus on lowering synthesis costs, increasing recycling efficiency, and designing derivatives with reduced environmental impact. Multinational teams evaluate new uses in supercapacitor electrolytes, CO2 capture, and biotechnology. Efforts to understand structure-property relationships reveal how tuning the hydroxyethyl side group changes performance, which helps chemists adapt the molecule for new industrial needs. Start-ups and university consortia push for scale-up methods that match both sustainability and regulatory drivers, hoping to lower barriers for customers in regulated sectors.
Toxicity Research
Most toxicity studies flag the main risk as release of fluoride ions under acidic or thermal extremes, a property linked to the PF6- group. At ordinary temperatures, the compound shows low acute toxicity, but chronic effects require more research. Cell culture studies point to low mutagenicity and no significant DNA binding, but tests do report moderate cytotoxicity at high concentrations or after long-term exposure. Wastewater or accidental spills require careful treatment to prevent environmental harm, and incineration remains a preferred disposal route for contaminated rags or containers. Governments and safety boards call for more in vivo testing, especially for new ionic liquids with similar PF6- content.
Future Prospects
Industry and academia watch this compound for new opportunities and challenges. Battery and fuel cell developers invest in ionic liquid electrolytes to break free from flammable carbonates and improve cycle stability. Separations engineers anticipate custom blends for rare earth or precious metal recovery. Pharmacology circles wonder if tailored ionic liquids like this one can modernize drug delivery or extraction in green pharmaceutical processing. Supply chains still face price pressure due to raw material and purification costs, but process intensification and recycling advancements aim to smooth out volatility. Governments and environmental organizations continue studying both benefit and risk, hoping to set smarter regulatory standards that foster responsible broader adoption of ionic liquids, including 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate.
What’s Going On with This Chemical?
Sometimes, science seems to invent ingredients just to test your spelling skills. Still, 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate stands out not for its tongue-twisting name, but for the role it plays in modern chemistry and industry. If you work in a startup lab or a process plant, you might spot it on a supply list—or at least you’d notice the label is impossible to pronounce.
The Real Magic: Ionic Liquids at Work
This compound falls under the family of ionic liquids. Scientists like these compounds because they stay liquid at room temperature. That opens up handy shortcuts during chemical reactions. I’ve seen custom-built reactors, where switching out a conventional solvent for an ionic liquid, like this one, changed how quickly or cleanly a reaction happened. There’s less fuss with pressure and temperature, and the process often becomes safer.
Cutting Down Waste in Green Chemistry
Chemists everywhere look for ways to stop filling up tanks with harmful waste. Ionic liquids offer a real shot at greener manufacturing. Unlike a flammable solvent, this chemical won’t light up near a Bunsen burner, and it sticks around after the job’s done for easy recycling. I appreciate seeing teams who want both good results and less clean-up, which this compound helps pull off.
Improving Battery and Electrochemical Tech
Battery researchers keep chasing longer life and better safety. Regular liquid electrolytes in lithium-ion batteries sometimes catch fire. Replacing them with 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate helps cut down the risk. I've watched engineers in test labs use variations of this salt to keep batteries cooler and last longer, especially in demanding gear like power tools or smart sensors.
Impact on Chemical Synthesis
Ask anyone doing organic synthesis, and you’ll hear gripes about tough-to-control reactions. This compound creates an environment that helps certain reactions, especially those involving metals or tricky organic molecules. Instead of waiting hours for unpredictable yields, chemists working with this ionic liquid often reach the finish line a bit faster and with cleaner product. More efficient synthesis leads to cost savings and fewer headaches scaling up.
Tough Spots: Health and Handling Questions
Not everything about new chemicals is rosy. Any time a material like this enters wider use, those handling it want solid info—skin exposure, toxicity, environmental disposal. The word in industry magazines and from experience points to careful storage and use in closed systems. No splashing it onto gloves or down a drain. Each manufacturer owes their staff detailed protocols. Regulators also keep a sharp eye on these new solvents, and I’m glad to see more paperwork, not less, when safety is on the line.
Looking Ahead: Alternatives and Solutions
More researchers push for even friendlier solutions—both for people and the earth. Ongoing work explores whether you can tweak these ionic liquids for faster breakdown after use, or find versions made from renewable sources. I find it encouraging that the more these chemicals replace older, more hazardous solvents, the closer the industry moves toward truly sustainable routines. No single solution fits every situation, and that's OK. Progress comes through choices—incremental ones, like swapping in a safer solvent, and bold ones, like rethinking the whole chemistry of a process.
Daily Safety Starts with Good Habits
People who work in labs or industries know: even a small oversight can trigger costly mistakes or real dangers. Picture a warehouse storing hundreds of chemicals—think of the tight spaces, quick shifts, and constant movement. I’ve watched a simple labeling slip-up force a full evacuation. That anxiety sticks. So, the basic rule is, don’t let “basic” habits slip. Secure labeling, locked cabinets, clear access—no one outgrows these basics. If you cut corners, it could become a safety story told for years, but not for good reasons.
Temperature Controls Are Non-Negotiable
Some compounds break down or turn hazardous just from a warm afternoon or a cold snap. I’ve seen compounds meant for 2–8°C left at room temp, the batch spoiled before anyone noticed. You lose money, you lose trust, and you definitely lose more sleep. Simple habits—thermometers, checklists, backups—keep things predictable. Some sites even install alarmed sensors that alert via phone if things drift. Isn't it funny how a cheap thermometer can do more for reliability than fancy software? Keep temperature logs honest by getting the team to sign off through the day, not just on paper later.
Moisture, Light, and Air: The Silent Wreckers
You know chemical containers with desiccant packets or amber bottles? It’s not overkill. Many compounds change, clump, or degrade with just a whiff of moisture or stray sunlight. I’ve seen humidity crumble what should have been reliable granules into useless sludge—impossible to recover and dangerous if reused. Airtight seals and keeping containers away from sunlight sound boring, but this is the cheapest insurance around. Some colleagues set up storage areas behind curtains or in rooms with no windows—it works, and it doesn’t break the budget. Give the team quick training; most people want to do things right, but shortcuts sneak in when things aren’t clear.
Compatibility: It’s Never “Just Store It Anywhere”
The job gets trickier with chemicals that react badly to common substances—acids stored near bases, oxidizers near organics, or volatile solvents mixing with incompatible materials. I once worked a shift where a supplier put flammable and corrosive containers side by side. We caught it, but the potential for fire was real, and it rattled everyone. The solution isn’t better signs but better habits: regular inventory checks, separate shelving, and never mixing old and new stock. Organize spaces by hazard, not just by convenience. Set clear rules and get leadership to back them—no one wants to challenge a supervisor, but safety wins these debates.
Preparing for the Worst, Even If It Never Happens
You never want to need the spill kit or eye wash station, but having them where people reach them is the easiest way to avoid panic. Keep spill absorbents, goggles, gloves, and emergency contacts posted and kept up to date, even if it means repeating drills more often than folks enjoy. I’ve seen a team ignore drills until the day something spilled—a little preparedness cut the chaos in half. And as for fire, don’t overlook routine extinguisher checks; one empty canister is all it takes for an accident to get out of hand.
Shared Responsibility
Management sets the tone, but it’s everyone’s job to stick to standards. I always respected workplaces where people spoke up—not just because it kept us safe, but because it showed trust. A good culture means nothing if people feel shy about raising a hand. Give open space for questions, encourage reporting, and celebrate good practice, not just results. Safe storage and handling aren’t glamorous, and they rarely get plaudits, but in the long run, they keep teams working and communities out of harm’s way.
Facing Chemical Safety in Research and Industry
Chemistry brings curious blends of promise and risk. In the lab and on the production line, materials like 1-hydroxyethyl-2,3-dimethylimidazolium hexafluorophosphate often sit at the center of innovation. This mouthful of a compound belongs to a class known as ionic liquids. Researchers favor these for their unique abilities—like dissolving tough substances or hosting tricky reactions. The flip side? Not every laboratory or factory worker knows whether they’re handling something gentle or something with bite.
The Trouble with Ionic Liquids
Not every ionic liquid is born safe. Some are gentle, others can burn, irritate, or even impact the nervous system. With 1-hydroxyethyl-2,3-dimethylimidazolium hexafluorophosphate, risks show up on two fronts. The imidazolium part doesn't usually cause headlines for acute toxicity, but not all imidazolium compounds act the same in the body. The more worrying piece comes from hexafluorophosphate. When exposed to moisture, this anion can break down, releasing chemicals like hydrofluoric acid—a name that makes even seasoned chemists take a deep breath. Hydrofluoric acid doesn’t play nice with skin, eyes, or lungs. One splash or whiff can lead to corrosive burns or, if not treated quickly, organ damage.
Hazard Lives in the Details
Public data for this specific ionic liquid doesn’t fill every blank. Most safety sheets, though, urge handling in a fume hood, avoiding skin contact, and wearing gloves and goggles. Toxicity studies on close relatives suggest potential for skin irritation, and inhalation might harm the respiratory tract over time. And any ionic liquid ending up in water could create headaches for fish or aquatic life—lab spills rarely stay behind closed doors forever. Even small discharges can upset rivers or drainage systems, especially when chemicals break down into even nastier bits.
My Time in the Lab
Personal experience counts for something on this front. Years ago, I worked with an imidazolium ionic liquid that, at first glance, looked as innocent as cooking oil. A minuscule spill reached my glove—not bare skin—but an hour later, my hand tingled and stung. The lab manager didn’t waste time, discarding the gloves and washing with soap and water. Sometimes, risk sneaks in quietly, only showing up when you feel a sting or an itch. This memory sticks, reminding me that even chemicals with unassuming names deserve respect—especially when people might not know the full story on toxicity or long-term health effects.
Practical Choices for Safer Chemistry
Working with chemicals that show even a shadow of hazard means putting real effort into safety. That looks like using gloves, goggles, and reliable ventilation. Training doesn’t work if it’s just a box on a checklist—everyone deserves clear facts about what they’re handling. Labels and Safety Data Sheets (SDS) must stay up-to-date, and spill kits should never gather dust on a shelf. On the industry side, engineers can look for greener, less toxic solvents and push for thorough study of new materials before sending them out into the world. Science doesn’t stop, but progress can make room for fewer headaches and safer hands.
A Call for Better Data and Vigilance
Fresh research fills gaps in our knowledge, but until the story of 1-hydroxyethyl-2,3-dimethylimidazolium hexafluorophosphate is complete, caution wins out. Nobody wants to learn through injury or accident. More studies, along with solid risk communication, will carve out a safer path for everyone working with advanced materials like this one.
Why Knowing the Structure Matters
Staring at a product label, you might see a slick chemical name like sodium laureth sulfate or ascorbic acid. For many, this string of letters means almost nothing. Some shrug, some worry, and some start searching online forums. Folks deserve to know what's inside what they buy and use. The chemical structure—how atoms fit together in a molecule—can shift how safe or effective a product turns out to be. People with allergies, for example, could avoid a rough reaction if they simply saw the right formula spelled out clearly on the packaging.
Having trust in what we eat, spread on our skin, or clean our homes with, starts with clear information. Taking acetaminophen as an example, its chemical formula, C8H9NO2, tells chemists where those carbon, hydrogen, nitrogen, and oxygen atoms sit. Shifting even a single atom leads to different results in the body. That’s not trivia. Acetaminophen relieves pain, but its close cousins might do nothing or even cause harm.
The Trouble With Generic Labels
A product can say “contains fragrance” or “surfactant,” yet tens or hundreds of chemicals might fall under that umbrella. Not every fragrance compound causes trouble for everyone, but some cause headaches or even asthma for a few. Fully listing chemical formulas lets scientists and health officials track down which ingredients spark issues. Parents with kids who react to certain preservatives don’t want marketing fluff—they want facts they can use. The formula, laid out in black and white, acts almost like a fingerprint.
Manufacturers often claim trade secrets, worried their precise blend might be copied. At the same time, public confidence rides on clear information and the ability to double-check what’s inside. Trust takes a hit when companies hide behind vague ingredient lists. In my experience, the frustration from families dealing with food allergies or sensitive skin often boils down to the lack of simple chemical truth in product details.
Steps Toward Greater Transparency
Countries like the United States require ingredient lists, but only in plain names—not always the detailed chemical structure or formula. Regulatory bodies in the European Union and Japan sometimes push harder for specifics, especially in cosmetics and food additives. Change moves slowly, often blocked by industry politics and cost. Still, some brands now post full chemical details on their websites, signaling respect for customer curiosity and safety. That sets a solid example.
Digital tools could bridge the gap. Imagine scanning a barcode in the grocery store and instantly seeing each ingredient’s chemical formula and a quick plain-English description. Plenty of us now check apps for reviews and dietary labels. Adding structural data points—like molecular structure diagrams or CAS numbers—could help scientists, healthcare workers, and curious shoppers alike.
Food, medicine, cleaning sprays—these touch our families daily. Offering up the chemical formula means companies have nothing to hide, and buyers get the choice to decide for themselves. Knowledge shapes healthier, happier habits. Keeping chemical structures transparent turns that knowledge into power in the hands of ordinary people.
Why It Matters
Tucked away in research labs and specialty industries, 1-Hydroxyethyl-2,3-Dimethylimidazolium Hexafluorophosphate plays its part in green chemistry, energy storage, or catalysis. Not everybody thinks about what happens to this ionic liquid once a project wraps up, but disposal isn’t an afterthought. It’s wrapped in science, safety, and stewardship. It touches the worker in the lab coat, the custodian at the hazardous waste site, and—ultimately—the river, ground, or air downstream.
What Makes This Compound Tricky
This chemical sports a double challenge. The imidazolium base can pose toxicity risks, and its hexafluorophosphate counterion holds fluoride, bringing risks of environmental contamination. Mishandling means trouble for aquatic life, damage to soil, or even toxic off-gassing in the wrong conditions.
Scientists have seen certain ionic liquids disrupt biological cells, especially aquatic organisms. Many assume ionic liquids break down easily. Some do, many don’t—especially those containing fluorinated anions like hexafluorophosphate.
Real-World Protocols
Colleagues in research and industry share a standard mantra: treat waste like it matters. That starts at collection. Specialized waste containers, marked and kept away from other solvents, keep exposure risks down. Gloves and goggles aren’t just for dramatic effect in the movies—they shield from spills and splashes, especially during transfer.
Once waste builds up, it doesn’t disappear “down the drain” in responsible labs. Environmental guidelines—from the EPA to European REACH standards—prohibit sending this stuff to municipal waterways or regular landfills. Many research institutions require transfer to licensed hazardous waste contractors. Incineration at temperatures above 1,100°C, with proper scrubbing for toxic gases, breaks the compound down safely. I once toured a waste facility where you could see their control room bristling with monitors, all focused on scrubbing fluorine emissions after incineration.
Hydrolysis can’t be relied on. Hexafluorophosphate reacts with water, sometimes producing hydrofluoric acid—a highly corrosive product, not easily handled outside of specially equipped facilities. Even small spills see strict clean-up protocols, including neutralization and vacuum systems with HEPA and acid gas filters.
The Cost of Cutting Corners
Labs dumping this chemical carelessly risk fines, shutdowns, public health alerts, and environmental damage. In one case, an accidental discharge into a local waterway led to fish kills and a six-figure cleanup bill for the university. It wasn’t just about rules—it was about stewardship, respect, and the consequences of ignoring the chain of custody.
Better Solutions on the Horizon
Some groups focus on developing “greener” ionic liquids with degradable anions or less toxic profiles. Until those are mainstream, safe handling and responsible disposal demand discipline and institutional support. Digital inventory tracking, training refreshers for waste streams, and tighter vendor takebacks all help.
The people who handle ionic liquids every day drive safer practices. The lesson: treat chemical waste not as an afterthought, but as a core part of science. Disposal shapes not just the lab, but the world outside its walls.