1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate: Insightful Commentary
Historical Development
Some chemicals grab the attention not from random novelty, but because a real hurdle in labs and industry creates demand. 1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate emerged from a series of efforts among chemists looking to wrest better performance out of task-specific ionic liquids. Looking back, early work on imidazolium-based ionic salts exploded after the 1990s, as scientists realized their conductivity, thermal stability, and negligible volatility changed the rules for catalysis, electrochemistry, even green solvents. The road to more advanced derivatives wound through dozens of tweaks, with research focusing on stability under operational stress, resistance to hydrolysis, and tuneable solvation profiles. The work that led to the hydroxyethyl variant involved balancing the desire for strong ionic interactions with structural modifications that allow better solubility or functional selectivity, pushing past basic alkyl-substituted salts.
Product Overview
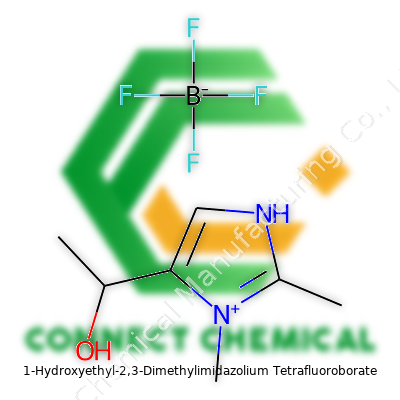
1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate offers a mix of unique attributes. Its imidazolium core gets tailored with two methyl groups on the 2 and 3 positions, a hydroxyethyl chain extending from the nitrogen atom, and paired with the robust tetrafluoroborate anion. This combination extends thermal and electrochemical stability compared to early ionic liquids, and gives chemists new tools for design and integration. Chemists came to rely on the product for applications ranging from reaction media in hydrogenation to electrolytes in energy devices.
Physical & Chemical Properties
This compound carries a dense, highly polar structure. In most conditions, it remains a viscous, colorless liquid, with a melting point comfortably below room temperature—one hallmark of ionic liquids. Its low volatility brings safer handling compared to volatile organics. Tetrafluoroborate anion provides chemical resistance and keeps hydrolysis to a minimum. Conductivity measures well above traditional organic solvents, making the compound well-suited for use in batteries, supercapacitors, and electrochemical cells. Its hydroxyethyl branch increases hydrogen-bonding interactions, which assists with solubility for a range of inorganic and organic substrates, and even stabilizes transition-state complexes during catalysis.
Technical Specifications & Labeling
Producers typically supply this salt with a purity of 98% or higher. Any trace water or chlorides prompt problems in sensitive applications, so specifications will always highlight water content, halide residuals, and even color stability under ambient light. Labeling gives a clear, unambiguous chemical name; batch number; storage recommendations—often in dry, air-tight vessels; and hazard symbols related to both the cation and tetrafluoroborate’s toxicological profile. For quality-conscious buyers, some producers offer a certificate of analysis with GC or NMR trace confirmation. Sizes start from lab aliquots of 5g or 10g, scaling up to industrial lots for manufacturers.
Preparation Method
Synthesis begins with 2,3-dimethylimidazole, itself made through methylation protocols familiar to anyone in bench chemistry. Alkylation with 2-chloroethanol under phase-transfer catalysis introduces the hydroxyethyl moiety, yielding the desired imidazolium hydroxide. Anion exchange with sodium tetrafluoroborate completes the process, precipitating sodium chloride for easy removal through filtration and vacuum drying. Many labs deploy strict anhydrous conditions at this step, because water contamination triggers hydrolysis of the tetrafluoroborate, leading to HF formation—a corrosive and persistent safety hazard. Every batch requires rigorous post-synthetic purification, and glass equipment due to HF’s etching capacity.
Chemical Reactions & Modifications
1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate has a structure that opens up targeted modifications. The hydroxyethyl chain supports further derivatization—such as esterification with carboxylic acids to fit specialized roles. Its imidazolium ring can participate in N-heterocyclic carbene (NHC) formation, with value for catalysis. In electrochemistry, researchers harness the strong ionic nature to stabilize radical intermediates or shuttle ions across barriers. Because of the strong hydrogen-bonding, this salt works well as a cosolvent for polar organic reactions, and it moderates acidity, especially in Brønsted-acid catalyzed transformations. Reactivity differs from alkyl imidazolium analogs, showing new selectivity profiles in epoxidation and cycloaddition reactions.
Synonyms & Product Names
Companies and research groups refer to the salt under several names—often abbreviating the lengthy nomenclature. “Hydroxyethyl-dimethylimidazolium tetrafluoroborate” appears in supplier catalogs. Literature abbreviations like [HOEtDMIm][BF4] clarify the structure in research papers and databases. Some commercial offerings tag it as “HE-DMIm BF4” or use numbers for methyl positions, leading to designations like 1-(2-Hydroxyethyl)-2,3-dimethylimidazolium tetrafluoroborate. This maze of terms can confuse a newcomer, so research chemists watch for structure-based names and match them back to CAS registry numbers.
Safety & Operational Standards
Safety develops from real risk. This compound brings low volatility, which cuts inhalation hazards, but the tetrafluoroborate counterion poses a persistent hydrolysis risk—HF formation burns skin, disrupts cell membranes, and etches glass. Training and personal protective equipment become non-negotiable: gloves, goggles, and fume hoods provide a basic suite of safeguards. Any container left open on the bench picks up moisture from the air, creating a slow and nearly invisible buildup of hazardous residues. Disposal involves neutralizing fluoride and consulting local regulations, as regulatory agencies flag both imidazolium derivatives and tetrafluoroborate salts for environmental monitoring. Technical sheets from leading vendors unpack these risks with up-to-date information for process engineers and laboratory staff. My own experience stresses repeated safety audits and regular refresher courses for all personnel.
Application Area
Application areas stretch across several industries. Electrochemists use this ionic liquid as an electrolyte in lithium-ion cells and supercapacitors, where its conductivity and thermal stability offer improved cycling performance and safety profiles. Catalysis labs turn to this salt for reactions requiring careful control over polarity and ionic strength, and in biotransformations it stabilizes enzyme activity under stress. In separation science, the unique solvation characteristics allow for the selective extraction of organics, metals, and rare earths—something I’ve seen firsthand when collaborating on waste remediation projects. Industrial adoption depends on scale, cost, and the regulatory environment, but the growing demand for cleaner, sustainable processing gives these ionic liquids a front-row seat.
Research & Development
Research never stands still with this family of chemicals. A steady stream of papers investigates how structural tweaks—longer alkyl tails, chiral centers—change not just physical properties but also reactivity and downstream impacts. Scientists probe ion transport, viscosity, and stability with advanced techniques like NMR, EPR, and computational modeling. Collaborations between academic labs and industrial users drive the optimization of electrolytes in batteries and fuel cells, where tiny changes in moisture content or ion pairing ripple into performance metrics. The move toward fine-tuned, task-specific ionic liquids accelerates as machine learning and high-throughput screening suggest new candidates faster than traditional bench methods. From my angle in applied chemistry, this intersection of theory and practical need proves the most exciting—problems in scale-up, purity control, and waste handling all get rigorous attention.
Toxicity Research
Toxicity can’t play second fiddle in any development cycle. Regulatory agencies and toxicologists have examined the imidazolium cation for cytotoxicity, biodegradation, and environmental persistence. Results indicate moderate acute toxicity in aquatic organisms and some persistence in soils. The hydroxyethyl group may impact cell membrane behavior, which raises new questions in bioaccumulation research. The tetrafluoroborate anion holds a stubborn resistance to hydrolysis in dry environments, but given enough time or exposure to moisture, it degrades to fluoride ions—each linked to bone toxicity and damage to aquatic ecosystems. Responsible vendors conduct full lifecycle assessments, and academic collaborations examine every link in the chain from production waste to end-user handling. The stakes run higher as more sectors adopt ionic liquids, and more rigorous, long-term toxicology data needs prioritizing.
Future Prospects
Future prospects revolve around a blend of opportunity and responsibility. Engineers chase new applications in high-performance batteries, industrial separations, and catalysis, where the ability to design a solvent or electrolyte from scratch promises enormous technical wins. Companies watch cost curves, regulatory guidance, and public perception, since new chemical introductions often spark debate over environmental impact. Research for safer counterions, faster biodegradation, and reusability gets funding from private and public sectors alike. The next generation of ionic liquids may stem from the knowledge base built on compounds like 1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate. In my experience, every advance raises two new questions: how to use the material responsibly, and how to manage the legacy footprint. Scientists, policymakers, and industry must align on health, safety, and life-cycle planning well before market adoption to keep these options on the table for future technology.
Unlocking the Role of Modern Ionic Liquids
Ask most folks about ionic liquids, and the conversation might stall. Not in research labs though. Chemists have explored thousands of these compounds. 1-Hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate carries a name that’s a mouthful, but its value becomes clear in action. It steps up as an ionic liquid—meaning it stays liquid below 100°C and does not have the volatility of old-school solvents. That matters for both safety and practicality, especially when volatile solvents raise issues in crowded labs and bigger plants.
How Researchers Put It to Work
I watched colleagues in green chemistry roll out this compound for its ability to dissolve challenging materials. There’s no point fighting with solvents that evaporate or catch fire too fast. This ionic liquid helps with dissolving cellulose and other biomass, which usually fight tooth and nail against traditional solvents. So it gives a leg up in the race for sustainable processes—think making biofuels or bioplastics.
There’s another angle—electrochemistry. The world leans toward energy storage. Batteries and supercapacitors see no shortage of work these days. Here, this compound slots in as an electrolyte. With a wide electrochemical window, it deals well with the stronger demands of next-gen devices. Stable and non-volatile means better safety for battery labs and test benches. The performance boost is real. Research papers show improved lithium-ion battery cycle life with ionic liquid electrolytes like this one, compared to old standard ones.
Catalysis and Green Synthesis
Nobody gets excited cleaning up toxic waste after a reaction. That’s one reason ionic liquids gain traction. Scientists use 1-hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate in catalysis, especially for coupling reactions—joining small molecules into more complex ones, often used in pharmaceuticals. This ionic liquid acts as both solvent and facilitator, which reduces waste. Efficiency jumps, too, based on studies from several journals. Fewer side products make life easier in the lab.
Handling this compound always seems less nerve-wracking compared to volatile organics. No hard smells, no headaches, and it sticks around in the flask. My experience says the clean-up is easier, cutting down on hazardous chemical waste.
The Environmental and Health Side
Chemistry circles talk about “greening” the lab. Ionic liquids aren’t perfect, but they step up compared to older options. 1-hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate does not vaporize into the air, limiting exposure risk for lab techs and the environment. Still, no chemical is truly without baggage. Disposal takes care, especially since the tetrafluoroborate part can pose aquatic toxicity risks if labs treat waste casually.
Where the Technology Heads Next
Academic journals report new uses almost every year. Right now, folks look toward using ionic liquids as designer solvents for tough industrial processes—such as CO2 capture or metal processing. The customizability stands out. Tweaking a side group can change how the solvent works, opening new options for hard-to-solve problems in renewable energy and pharmaceuticals.
Progress over the next decade may depend on lower costs and easier recycling. Some labs and companies recycle these liquids, using filters and distillation. Regulatory bodies keep an eye on new chemicals, encouraging more data on safety and environmental impact.
Every time I see 1-hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate show up in a research report, it’s often tied to better results with less environmental fuss. That’s the direction science wants to move: real-world gains with lower trade-offs.
Structure That Sparks Curiosity
Chemists always chase ways to make reactions faster, cleaner, and more predictable. Many of us, seeing an ionic liquid like 1-Hydroxyethyl-2,3-Dimethylimidazolium tetrafluoroborate, perk up. Its name looks hefty, but break it down and the structure actually feels approachable for anyone who ever doodled rings in a lab notebook.
The Molecular Blueprint
The chemical formula speaks volumes: C7H13N2O+ BF4-. This compound centers around an imidazolium ring—a five-membered structure with two nitrogens, familiar to many who study biochemistry or medicinal chemistry. At the 2- and 3-positions, methyl groups take root, while the 1-position features a hydroxyethyl group. That addition gives the molecule a handle for hydrogen bonding and helps it dissolve a diverse range of materials.
The counterion, tetrafluoroborate (BF4-), balances the positive charge. This part doesn’t just fade into the background. Scientists value tetrafluoroborate because it rarely gets involved in side reactions and brings stability, especially at high temperatures or when strong acids and bases show up in a reaction mix.
Why This Structure Matters for Real Work
Lab experience shows that swapping out the side chains on imidazolium rings tweaks how these liquids behave. The hydroxyethyl side chain brings a touch of polarity and encourages mixing with water and alcohols. That’s important to my colleagues who rely on these liquids to dissolve organic and inorganic materials that don’t otherwise want to blend. It means cleaner work, less contamination, and fewer surprises.
Green chemistry gets a boost from these structures. I remember trying to separate out metal catalysts from old reaction waste. Ionic liquids like this one make recycling precious metals much more realistic. Instead of chasing solvent vapors or running lengthy purifications, I could use the unique layering and solubility properties to pull valued material straight from the reaction, dramatically cutting down on waste streams the lab has to manage.
The Science Leads to Real-World Solutions
In practice, the key strengths of 1-Hydroxyethyl-2,3-Dimethylimidazolium tetrafluoroborate—thermal stability, non-volatility, and broad solvent compatibility—set the table for safer and greener labs. These features mean less risk for accidents and easier containment when compared to common organic solvents.
Regulators, companies, and universities all want to keep unnecessary hazards away from workers and the environment. Adopting liquids like this ionic compound could shrink reliance on classic but dangerous organic solvents. Think of the reductions in lab fires, ground contamination, or costly disposal fees for solvents that stick around in groundwater. The structure’s very design—polar yet stable, big enough to dissolve complex molecules—offers a safer path forward for labs and factories without sacrificing chemical flexibility.
Looking Ahead
Wider use of molecules with this type of structure might help chemists push into fields like energy storage, CO2 capture, or safer pharmaceuticals production. There’s a lot to learn from imidazolium-based ionic liquids, and every time new side chains get attached or novel anions get paired, fresh opportunities emerge. Focusing on properties rooted in solid molecular science keeps research safe, efficient, and environmentally aware.
A Look at the Risks and Responsible Handling
I’ve spent a lot of time shuffling between various labs—sometimes with chemicals much less forgiving than table salt. The name 1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate sounds complicated, but like many ionic liquids, it draws interest for unique uses, from solvent work to batteries. Still, just because something gets a lot of buzz in the research world, no one should shrug off the real risks it poses.
The main concern is not only its chemical complexity but also the reactivity that comes with the tetrafluoroborate part. Ionic liquids built from imidazolium cores often carry the reputation for low volatility, which helps keep inhalation risk down compared to volatile solvents like acetone or ether. Don’t let that fool anyone into getting casual, though.
The tetrafluoroborate anion can react with water to release toxic hydrogen fluoride gas, especially if there’s enough moisture around or if the ionic liquid ends up exposed to acids. Even trace amounts of water or the naturally humid air in many labs are enough to start that slow, hidden process. I’ve seen first-year researchers forget gloves after “just refilling pipettes,” thinking a spill on skin is fine if it feels oily and not burning. That’s a myth. Many ionic liquids, including this one, sneak through gloves or break down skin barriers, and you often won’t feel the damage starting until it’s too late — especially with risks of chemical burns or deep tissue injuries from hydrogen fluoride.
Gloves, goggles, and full lab coats matter here. Nitrile gloves work better than latex for chemical resistance, and I always check the safety data sheet, since some chemicals chew through gloves fast. The fumes might not be obvious, but adding a chemical fume hood keeps the risk of accidental gas release lower. Experience taught me that spills happen, no matter how careful you feel. It pays to know where the nearest eyewash and shower stations sit — and to practice getting to them with your eyes closed.
In practice, storage and disposal decisions often separate seasoned chemists from amateurs. Anyone storing tetrafluoroborate salts in a humid shop or letting waste sit “just until the end of the week” is risking more than messy cleanup. Closed, clearly labeled containers kept in a dry space, outside of direct heat or sunlight, cut down both accidental mixing and decomposition risk. Disposal deserves real attention too, working with qualified chemical waste teams rather than pouring leftovers down the drain. Cities have paid big costs after improper fluorinated waste ended up in water treatment facilities.
It’s tempting to chase the promise of a new material, but safety culture underpins all good science. I’ve lost count of times I saw people get excited about performance or a new reaction—only to cut corners because things looked clear and didn’t smell. 1-Hydroxyethyl-2,3-Dimethylimidazolium Tetrafluoroborate brings opportunities, but safe handling protects not just the individual, but everyone else sharing space as well. Preparing for accidents, respecting the risks of both toxicity and reactivity, and following both written protocols and gut-level safety habits keep labs incident-free, and work humming along for the next day.
Understanding the Chemical
1-Hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate brings potential as an ionic liquid for green chemistry projects, battery electrolytes, and certain catalytic reactions. Anyone who works around specialty chemicals knows that storage means a lot more than just finding space on a shelf. With compounds like this, overlooked details can bite you later. I learned to respect shelf-stable claims only after watching a careless storage setup lead to slow leaks and label fades during a long, humid summer. This stuff carries both an organic structure and an inorganic anion, which can shift its behavior under different conditions.
Why Storage Matters with Ionic Liquids
Many people think that room temperature storage cuts it, especially for salts that seem solid and non-volatile. But even small errors can lead to degraded samples or even safety hazards. With tetrafluoroborate salts, moisture comes as the top enemy. The BF4- anion reacts with water, sometimes releasing toxic gases such as boron trifluoride and hydrogen fluoride. Hidden water in the air can creep in, unnoticed, until you pop open the bottle or pipette and catch a whiff of something strange.
Moisture: Hidden Issue in Labs and Storage Rooms
Even if the bottle seals well now, never underestimate the sponginess of cardboard boxes or humidity that drifts through a poorly ventilated room. Desiccators work well for smaller amounts. Larger drums benefit from moisture-barrier bags with denser walls, plus silica gel packs tucked in for insurance. Chemical storage cabinets with humidity controls make life easier, but few labs have enough budget or space for everyone to get one. Nobody likes opening what they expect to be a dry sample, only to discover sludge at the bottom or a changed smell after a few weeks of a rainy season.
Temperature: Not Just for Comfort
This chemical holds up at room temperature if kept dry, but routine lab environments swing hotter or colder based on season, sun, or HVAC breakdowns. High heat speeds up chemical reactions and can increase vapor pressure, which can stress containers, especially if they are already old or the screws aren't set tightly. I learned to keep sensitive bottles in the coolest, most stable spot of the lab away from sunlight or heating pipes. Refrigerator storage does the trick for some, as long as condensation gets controlled. Otherwise, opening a cold bottle in the wrong environment invites instant moisture.
Container Selection: An Overlooked Protector
Glass with a tight, chemical-resistant screw cap shields the chemical from air, light, and accidental spills. Plastics may work for temporary transfer, but avoid long-term storage in polyethylene or low-grade plastics because of slow diffusion of gases or chemicals. Dating every sample, adding hazard warnings, and using secondary containment trays helped me dodge headaches after someone else’s unlabeled spill made for a sticky cleanup years ago.
Labeling for Long-Term Safety
If a sample outlives people’s memories or job titles, labeling steps up as the only thing standing between safety and disaster. Waterproof labels and ink, updated hazard information, and a clear purchase or synthesis date save both time and safety. Every year, check for leaks, odd color changes, or crust build-up. Proper logs backtrack any problem to its source fast.
Simple Steps, Big Difference
Dry, cool, and airtight: these three targets cut most problems short. My early mistakes taught me not to trust best guesses or old stories. Each new batch, supplier, or container can hide surprises. No wonder the most careful chemists I’ve met keep checklists, assign people to check chemical rooms, and double-lock high-risk bottles. Wise storage beats cleanup or exposure every time.
Understanding its Place in Modern Chemistry
Chemists tend to get excited with a substance like 1-Hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate. For those new to it, this compound brings a unique mix of stability and reactivity, a combo that always grabs the attention of researchers. Ionic liquids often thrive when they show low volatility, thermal stability, and an ability to dissolve a wide swath of molecules. This one checks a lot of those boxes.
Properties that Matter
I've worked with several ionic liquids in the lab, and the best ones hold up under stress. 1-Hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate doesn’t flinch when the heat gets turned up. Its negligible vapor pressure keeps laboratory air clean, and its capacity to withstand high temperatures heads off safety worries. Handling a liquid without that sharp, solvent stink, and with little fire risk, makes daily routines safer.
Another thing worth mentioning: its imidazolium structure. This shape gives real versatility, helping the compound snuggle up to both organic molecules and metals. It goes beyond theory—this versatility lets researchers use it as a solvent for green chemistry, or even in processes that demand precise separation, like extracting rare earth metals.
Meeting Industrial Challenges
Industries have pushed for molecules that help cut down hazardous waste. Here, ionic liquids have gathered a following. 1-Hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate provides a shot at safer processes. Compared to old-school organic solvents, there’s less risk of harmful vapor exposure. Real-world data shows that ionic liquids can cut emissions and make recycling easy, which big factories take seriously as environmental rules grow strict.
There’s talk about cost, though. These materials don’t come cheap at scale, and every production manager weighs the expense. Companies look at the full lifecycle—what it costs to buy, what it saves down the line, how easy it is to handle waste. From experience, clever recovery systems and reuse can draw down those long-term costs. It’s not easy to justify budgets up front, but tighter regulations and cleaner technologies push more firms to invest.
Environmental and Health Considerations
Ionic liquids get a green reputation, but it’s not a free ride. Tetrafluoroborate, for example, can break apart in water, and you don’t want fluoride ions loose in rivers. Reports have flagged toxicity issues, pushing labs to check disposal programs twice. If a company plans to dump waste, it pays to test for breakdown byproducts and be up front with regulators.
Solutions and Progress
One answer involves beefing up waste treatment at the source. I’ve seen wastewater systems that capture fluorinated leftovers and neutralize them before they reach the pipes. Companies can partner with researchers to cook up alternative ionic liquids with less toxic pieces, a strategy that takes shape as more funding flows into sustainable chemistry. Chemists continue to tweak these imidazolium structures, hunting for options that boost safety without losing what made the original useful.
Applications for 1-hydroxyethyl-2,3-dimethylimidazolium tetrafluoroborate aren’t limited to a single corner of chemistry. Electrochemistry benefits from its ability to improve ion movements and boost battery technologies. Pharmaceutical processes can cut step counts or swap harsh solvents in favor of safer runs. Each success story starts with responsible handling and a commitment to tweaking formulas as new problems surface.