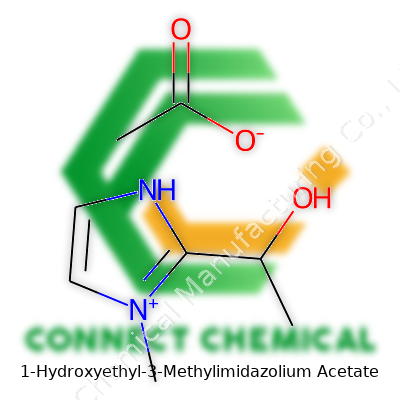
1-Hydroxyethyl-3-Methylimidazolium Acetate: Progress, Properties, and Future Direction
Historical Development
The field of ionic liquids has pushed chemistry into new territory. Among the most widely discussed is 1-hydroxyethyl-3-methylimidazolium acetate, a name that has landed in countless research papers since the early 2000s. Chemists searched for solvents that dodge problems like high vapor pressure and toxicity, especially as environmental standards climbed. These efforts led to the design of imidazolium-based ionic liquids. In academic circles and more specialized industries, this compound’s story runs parallel to the push for greener, safer solvents. The initial synthesis involved adapting reaction conditions tested for other ionic liquids, borrowing from methods used with traditional imidazolium systems and reacting derivatives to incorporate the hydroxyethyl group. Its trajectory has followed the rise of green chemistry movements and the urgent drive to update industrial solvent choices.
Product Overview
1-Hydroxyethyl-3-methylimidazolium acetate stands out as a hydrophilic ionic liquid. The molecule contains an imidazolium core, a hydroxyethyl chain, and an acetate counterion. The molecular design reduces volatility and offers unique solvation power. Rather than drifting into niche use, this compound quickly grabbed attention as a dissolving agent for cellulose, lignin, and other natural polymers. In a chemistry lab, storage vessels bearing its name now share shelf space with legacy solvents, reflecting its acceptance.
Physical & Chemical Properties
Its clear, colorless to pale yellow liquid appearance tells you something right away: this compound blends into its surroundings quietly. It resists evaporation, bringing boiling points well above ordinary organic solvents. Density usually sits between 1.1 and 1.2 g/cm³ at room temperature, and the viscosity stays moderate, which helps with handling in reaction setups. Solubility in water and other polar media simplifies clean-up and removal from reaction residues. The chemical stability holds steady under neutral and slightly basic conditions, though acidic extremes or high temperatures beyond 200°C start to break things down. The acetate anion endows mild basicity, making this liquid an effective medium for both synthetic and processing tasks.
Technical Specifications & Labeling
Suppliers ship it in high-density polyethylene bottles—sometimes amber glass for longer storage—to keep out light and moisture. Specifications run to purity above 98%, with moisture content often less than 0.5%. Product labels show the full systematic name, the CAS number for inventory control, recommended storage conditions (usually between 10°C and 25°C), and pictograms for handling. Batch records and certificates of analysis back up claims of purity and trace residue levels, such as halides or transition metals. This makes traceability a real possibility for buyers in regulated environments.
Preparation Method
The main route relies on quaternization and anion exchange. Synthesis kicks off by reacting 1-methylimidazole with 2-chloroethanol, yielding 1-hydroxyethyl-3-methylimidazolium chloride. Subsequent anion metathesis with sodium acetate produces the acetate salt, leaving sodium chloride as the byproduct. Purification goes through water washing, rotary evaporation, and sometimes vacuum drying. Troubles often center on getting rid of excess water and unreacted starting materials, since even 0.1% impurities can influence downstream uses. More sustainable variations swap in bio-based raw materials or tweak the workup to reduce waste, an evolution that mirrors wider trends in specialty chemical manufacturing.
Chemical Reactions & Modifications
Chemists value 1-hydroxyethyl-3-methylimidazolium acetate not just as a solvent but also as a reaction medium that can nudge certain transformations. In the realm of cellulose processing, it dissolves biomass at room temperature, paving the way for direct modification or derivatization. The hydroxyethyl side chain opens further chemical windows—esterification, etherification, and acylation reactions all proceed neatly under controlled conditions. In catalytic systems, this ionic liquid sometimes stabilizes reactive intermediates or changes selectivity paths. Researchers also report on pairing this ionic liquid with metal salts or organocatalysts, which can tune reaction outcomes for synthesis of fine chemicals or pharmaceutical intermediates.
Synonyms & Product Names
Literature refers to this compound in several ways: [HEMIM][OAc], 1-(2-Hydroxyethyl)-3-methylimidazolium acetate, or simply hydroxyethylmethylimidazolium acetate. Some suppliers include brand marks or abbreviation codes, such as HEMImAc, to identify variations produced in-house. The consistent styling in academic and patent publications keeps confusion in check among researchers and buyers. In certain markets, regulatory filings use either the full IUPAC name or an N- number from chemical inventories.
Safety & Operational Standards
On safety sheets, acute toxicity lands in the mild range, mainly as an irritant to skin and eyes. Standard lab gloves, splash goggles, and fume hoods form the first line of defense during use. Accidental spills tend to be easy to contain, thanks to low volatility and quick miscibility with water. Waste handling guidelines send bulk residues into solvent recycling streams, not into conventional drains, to stay in line with environmental codes. Operators should monitor for chronic exposure, though the compound’s bioaccumulation risk looks low based on early studies. Emergency first aid follows the protocols for mildly basic organic liquids. Fire risk almost never comes up in practice, as the flash point sits above 200°C.
Application Area
In industry, the main draw remains biomass processing—especially pulping and fractionation of lignocellulosic materials. Pilot plants use this ionic liquid to produce cellulose films and fibers with a lower greenhouse footprint than legacy processes. In lab research, it shows up in organic transformations, polymer synthesis, battery electrolyte development, and even enzyme stabilization studies. Some teams experiment with using this solvent for recycling rare earth elements from spent electronics, building on its unique extraction characteristics. Inside research groups, chemists find unexpected value by coupling the solvent with catalysts to dig at otherwise sluggish reactions. In all these cases, the compound’s broad solubilizing ability trumps that of classic organic solvents, and the added function of the hydroxyethyl group supports new reactivity.
Research & Development
Over the last decade, scientific interest poured into tuning the molecular structure, swapping different side chains, or switching the anion to build an entire library of new liquids. In my own readings, half the breakthroughs come from subtle shifts: adding bulk to the hydroxyethyl tail alters viscosity and changes how well the solvent interacts with tough substrates. Consortia between academic labs and green chemistry consortia document pilot trials at centimeter to kilogram scale. Researchers focus on improving recyclability, lowering synthesis energy demands, and predicting physicochemical behavior using advanced simulation software. Projects also target integrating this compound with biorefinery streams to valorize waste plant material—efforts that could change the economics of second-generation biofuels. Teams share findings through open-access journals and industry white papers, marking real progress in transparency and collaboration.
Toxicity Research
Toxicologists report that 1-hydroxyethyl-3-methylimidazolium acetate generally shows low acute toxicity in controlled studies, both in animal models and cellular assays. Chronic exposure data remain murkier, largely due to limited long-term studies. The acetate anion does increase the potential for mild skin or mucous membrane irritation, so workplace exposure limits draw from short-term animal studies rather than population-level epidemiology. In my own lab experience, even after regular use, there’s no sign of adverse effects when standard PPE stays in play. Environmental toxicology paints a slightly different picture: the compound degrades slowly in aquatic environments, though not as stubbornly as some legacy solvents. This triggers a push to develop enzymatic or microbial treatment systems to reduce persistence before discharge. Tracking these data matters, especially as regulatory agencies begin to scrutinize ionic liquids under revised chemical assessment regimes.
Future Prospects
Demand for green solvents keeps rising, especially across the biorefinery and materials science worlds. 1-hydroxyethyl-3-methylimidazolium acetate holds a front-line position in these transitions, offering a scalable alternative to both volatile and toxic solvents. As companies look for new ways to process agricultural waste or recycle plastics, this ionic liquid’s unique dissolving properties mean it is primed for practical expansion. Investment flows into tweaking the synthetic steps, scaling up production, and incorporating this family of solvents into new batteries, sensors, and even continuous-flow chemical reactors. If the next wave of regulation focuses more on life-cycle analysis and end-of-life management, research efforts need to dig deeper into reuse and cleaning systems, balancing operational value with downstream ecological impacts. Innovation in customizing the structure, boosting recycling rates, and integrating with renewable feedstocks shapes the conversation on what future chemistry looks like. Real progress will flow from ongoing partnerships between industry, academia, and regulators—not just from bench-scale triumphs, but from systems built to withstand the scrutiny of life outside the lab.
What Makes This Chemical Matter
Walking into a modern lab, not many bottles draw a second glance, but one labeled “1-Hydroxyethyl-3-Methylimidazolium Acetate” tends to get a nod from the folks who know cellulose or biofuel work. It sounds like a mouthful, but its impact in green chemistry circles is pretty real. Over the last decade, this ionic liquid has left a mark on researchers aiming to move away from old-school, pollutant-heavy solvents.
Why Cellulose Matters
Take wood pulp, paper waste, or even old cotton shirts—fibers stick together because of hydrogen bonds. Most solvents struggle to break those up. 1-Hydroxyethyl-3-Methylimidazolium Acetate steps in as one of the few that can dissolve cellulose without tearing it apart at the molecular level. That’s a huge deal. Dissolving cellulose opens up ways to make things like bio-based plastics, specialty fibers, and even new kinds of biodegradable packaging. The process taps into renewable resources, which is a key goal for industries looking to cut down plastic waste.
Biofuels and Breaking Barriers
For years, scientists ran into brick walls trying to turn plant material into fuel efficiently. The tough structure of lignocellulosic biomass doesn’t yield sugars easily. Using this ionic liquid, researchers break apart cellulose from raw plant material, making enzymatic conversion to sugars a lot easier. That’s an important step toward making affordable, renewable fuels. Thinking globally, the push to replace gasoline with plant-based fuels relies heavily on cost-effective, clean ways to crack open biomass. This chemical stands out for its ability to help unlock those sugars without the pollution headaches that traditional acids and bases give us.
Lab Work and Scale Challenges
Every time I’ve seen a student mix up a beaker of this ionic liquid, the same concerns pop up. Safety comes first; protective gloves are a must since many ionic liquids can cause skin irritation. Then there’s cost: large-scale work hammers budgets, since making ionic liquids in big volumes isn’t as cheap as older solvents. That limits real-world adoption outside high-value projects or research settings. Add in worries about long-term stability and disposal, and you see why some manufacturers are slow to embrace it, despite the clear environmental upside.
The Push for Greener Chemistry
One thing stands out—the labs always look for easier recycling methods for solvents like this one. If companies crack solvent recycling at an industry level, stronger adoption should follow. Since sustainability isn’t just about greener starting material, but also about every step along the way, groups are turning to closed-loop systems. That means using, cleaning, and reusing the same batch of solvent multiple times. Each cycle trims waste, saves cash, and prevents unnecessary disposal in the environment.
The Road Ahead
The attention this acetate gets from universities and companies points to a bigger trend. Using old plastics doesn’t feel right, given the mess piling up in oceans and dumps. If new chemicals offer a way to unlock plant fibers or turn waste into clean energy, it’s worth the effort. The science is there—what remains is shrinking costs, tackling safe handling, and keeping the process as clean as the chemistry itself. If those gaps close, 1-Hydroxyethyl-3-Methylimidazolium Acetate could shift from curiosity to cornerstone in sustainable manufacturing.
The Real-World Chemistry Experience
Lab work demands plenty of focus and respect for chemicals, even the so-called "greener" ones. 1-Hydroxyethyl-3-methylimidazolium acetate stands out in chemical circles as an ionic liquid used for dissolving cellulose, working in organic synthesis, even helping to extract bioactive compounds from plants. At first glance, its green chemistry label can be disarming, suggesting a softer risk profile than older solvents. Tempting as that sounds, nobody at the lab bench can count on safety without understanding a chemical’s risks.
What the Data Tells Us
This ionic liquid won attention for low volatility and apparent biodegradability. But science expects more than a marketing phrase. The chemical’s molecular structure—built from an imidazolium cation paired with acetate as anion—means it won’t easily evaporate into the air and fill the lab with odors. Yet, low vapor pressure doesn’t guarantee safety. Safety data sheets catalog it as an irritant, and experience lines up with that verdict. Even a mild solvent, in contact with skin or eyes, can sting or cause ongoing redness. I’ve seen folks develop irritation along glove cuffs after accidental spills—no one wants that sensation.
Peer-reviewed studies—like those in “Green Chemistry”—have checked toxicity. Some find moderate aquatic toxicity. Slightly safer than legacy organic solvents, maybe, but you won’t see it approved as a food additive or cosmetic ingredient. Chemical burns and delayed allergic reactions have popped up, especially with bare-skin handling over time. Inhalation risk looks very low, thanks to low volatility, yet splashes can still trigger eye and skin issues on contact.
Why Care About Proper Handling?
Trust in “greener” solvents grows in universities and biotech startups. Labs prefer fewer regulatory headaches, for sure. Still, plenty of people see protective gloves and goggles as overkill for something labeled “low hazard.” Reluctance grows in tight budgets and fast-moving workplaces, where safety slows things down. That trade-off can backfire. Across the globe, serious injuries often start from routine contact and small spills into gloves or onto sleeves. Extra minutes spent cleaning up or swapping out gloves looks minor compared to battling a persistent rash or worse.
Better Handling Strategies
Anyone working with this solvent can avoid unwanted exposure. Glove selection gets overlooked, but I’ve seen latex or nitrile gloves break down after direct exposure. Chemical-resistant gloves rated for ionic liquids—some thicker nitrile or sturdy neoprene—cope better. Face shields or snug goggles matter for weighing out materials or topping off flasks. Pouring from one vessel to another? Skip the rushed move; use a pipette or syringe.
Ventilation still helps. True, it doesn’t boil off like diethyl ether, but maintaining good airflow benefits everyone—accidental release of small particulates can still pose a hazard. Everyone in the lab should know spill procedures, no matter the solvent. Keep spill kits close, and check all safety data sheets instead of relying on memory or rumor. In my experience, training works—not as a boring formality, but as a real buffer between safe routine and the medical tent.
Why Stay Vigilant?
Advances in chemistry deliver new tools that sidestep the worst traits of legacy solvents. Still, even the friendliest chemical can cause trouble if folks relax basic lab discipline. Using 1-hydroxyethyl-3-methylimidazolium acetate safely means trusting the evidence, not the label. Protective gear, good habits, and reliable information form the real shield against accidents. If one practice defines a good lab, it’s keeping health and curiosity ahead of carelessness.
Peeling Back the Chemistry
1-Hydroxyethyl-3-methylimidazolium acetate shows a structure that’s familiar to folks who’ve spent time with ionic liquids. Chemistry textbooks paint this one with an imidazole ring—a five-membered ring with two nitrogen atoms—and some handy add-ons. One place on the ring holds a methyl group, and another spot features a hydroxyethyl chain. This ring, known as the imidazolium ring, forms the backbone. The positive charge gets centered at one of the nitrogen atoms on the ring after tacking on the hydroxyethyl and methyl groups. Then the acetate anion, with its carboxyl group, hangs around as the counterion. In formula terms, the cation looks like C6H11N2O+ and the acetate anion sits as C2H3O2-. That pairing matters—a lot—since the whole liquid owes its key traits to the marriage of those components.
Why Structures Like This Spark Curiosity
Grabbing hold of the chemical structure sheds light on what this ionic liquid gets up to in a lab or a factory. I’ve watched teams in sustainable engineering reach for that hydroxy group often: it changes hydrogen bonding enough to dissolve wood fibers in ways old-school solvents can't. Chemists see doors open with tasks like dissolving cellulose or helping enzymes stay active because the molecular landscape avoids harsh conditions. This one doesn’t boil or burn like nasty organic solvents, so labs avoid some of the safety headaches that used to pop up. Folks looking at green chemistry notice that the acetate, not some sneaky halide, tells you something about environmental goals.
Backed Up by Science
Peer-reviewed journals, like Green Chemistry and Journal of Physical Chemistry B, lay out plenty of evidence for why this compound stays relevant. Work led by scientists such as Robin Rogers has shown that imidazolium-based liquids, especially those with acetate anion, break down tough biopolymers like lignin and cellulose without the pollutant run-off linked to classic methods. Researchers punch in the SMILES string—CC1=CN=CN1CCO.[O-]C(=O)C—for this one on cheminformatics platforms, tracking reactivity and physical properties in big databases, echoing the trend toward open laboratory data.
Real Problems, Real Solutions
Not every breakthrough comes clean and easy. Industrial folks mention viscosity getting too high for pumping; it gums up reactors if nobody tweaks the formula. Toxicity still sits on the table—even green solvents see scrutiny under EPA and EU guidelines. Academic experience brings up issues scaling up: price spikes, batch variability, and leftover impurities walk hand-in-hand with even the most elegant chemistry lesson. Clear labeling and accessibility to scientific data support better decisions in industry and research, as transparency limits the risk of greenwashing.
Research teams now lean on computational work, modeling how tweaks to imidazolium or acetate flags shift toxicity and performance. If someone in a process development lab thinks about using 1-hydroxyethyl-3-methylimidazolium acetate, conversations about renewable sourcing and waste recovery keep from missing the bigger environmental picture. Everyone wins when improvements leave fewer scars on ecosystems. For students and chemists starting out, breaking down the structure by hand connects paper chemistry to the actions that drive responsible innovation.
Understanding the Chemical’s Nature
1-Hydroxyethyl-3-methylimidazolium acetate, often marked as an ionic liquid, draws attention for its ability to dissolve cellulose and other tricky biomaterials. Its stability and performance depend largely on how people handle and store it. From my time in chemical storage—across academic and commercial labs—it’s clear: chemical accidents or poor performance often stem from rushed or ill-informed storing habits. A few steps offer big payoffs in both safety and preserving the material’s qualities.
Humidity Risks and Water Absorption
This compound pulls in water from air faster than most might expect. Once the water content creeps up, the liquid’s physical and chemical properties begin to shift. I learned early on that letting these ionic liquids sit around with lids loose means a quick trip to ruined experiments and wasted money. People usually keep these types of chemicals in tightly sealed glass bottles. Sometimes, researchers toss in a layer of inert gas—often nitrogen or argon—before sealing. This helps push out moisture-loving air and holds off unwanted reactions.
Temperature and Light Exposure
The compound stays happiest in a cool, dark spot. Long-term storage above room temperature makes the risk of degradation climb higher. Sunlight warms bottles on window ledges and jumpstarts breakdown, especially for chemicals sensitive to oxygen or photons. A simple cabinet, kept below 25°C, keeps the risk low. In shared lab spaces, I have seen labels fade from sun-washed windows, but the real danger came from spoiled samples due to unnecessary exposure to bright light and summer heatwaves.
Material Compatibility
Many spend time checking bottle seals, but forget about what actually touches the liquid. Some plastics leach or soften, so glass holds its spot as the trusty choice. Teflon-lined caps can save a valuable sample from a vapor leak. When someone stores these ionic liquids in regular plastic, leaching odors or degraded container walls soon signal trouble. Simple choices—glass bottles, lined seal caps, no metal lids—get results.
Labeling and Documentation
A clear label keeps future confusion out of the way. Each bottle used in my workspaces shows name, preparation or purchase date, and sometimes water content. People leave samples in fridges without names, and months later, no one trusts the mystery bottle. A good inventory system on paper or digital logs helps scientists and safety officers track shelf life and order new material before a crunch arrives.
Emergency Preparedness and Risk Management
Even with careful storage, spills or leaks sometimes catch staff off guard. Spill kits and well-trained workers matter just as much as the right containers. I have watched junior staff clean up small leaks and learn the value of fast, correct response—preventing possible skin contact and larger issues. Training often relies on real-life stories to bring the message home: do things the right way, and bad days happen less often.
Summary of Best Practice
Storing 1-hydroxyethyl-3-methylimidazolium acetate takes discipline—tight seals, glass, darkness, and steady logs. The right care prevents unexpected costs, injuries, or time lost to ruined compounds. In well-run labs, these steps become habits, earning trust from researchers, manufacturers, and community safety officials alike.
The Challenge of Cellulose Solubility
Anyone with a hand in biomass processing or green chemistry eventually hits the cellulose wall. This tough, natural polymer binds itself with a network of hydrogen bonds. Typical water and most organic solvents simply bounce off. Dissolving cellulose effectively opens up possibilities for new materials—think advanced textiles, biodegradable plastics, and smarter paper. Yet, harsh chemicals like carbon disulfide often run the show, leaving environmental and safety concerns in their wake. So, the world turns its eyes to ionic liquids.
What Makes Ionic Liquids Tick?
Ionic liquids, especially ones built around imidazolium salts, catch the eye for their unmatched ability to disrupt those stubborn cellulose bonds. Take 1-Hydroxyethyl-3-Methylimidazolium Acetate, sometimes shortened as [HEMIM][OAc]. With its fluid nature, low vapor pressure, and structural tweakability, it promises a less toxic alternative. Some research, including a 2019 study out of the Technical University of Denmark, shows that this compound dissolves cellulose at levels rivaling and sometimes surpassing the popular 1-Butyl-3-methylimidazolium chloride.
Why Mixing Matters
Every chemist who has ever made a solution knows the value of time and temperature control. Trying to dissolve microcrystalline cellulose in [HEMIM][OAc] at temperatures above 80°C changes the speed of the process significantly. The acetate anion starts breaking down the hydrogen bonds. The hydroxyethyl group on the imidazolium ring amps up the hydrogen-bond donor capacity, pushing the process along. These technical details might sound dry, but they make the real-world difference between a batch that runs overnight or one that wraps by lunch.
Friendly to the Environment?
Traditional solvents like N-methylmorpholine N-oxide or carbon disulfide leave a heavyweight footprint. Accidents, toxicity, and waste disposal grow into big headaches. Ionic liquids such as [HEMIM][OAc] change that equation. They don’t evaporate easily, reducing air pollution. Recovery rates hover higher, so less solvent walks out the door after each run. Compared with older chemistries, labs have documented lower aquatic and human toxicity, although long-term degradation rates still need more independent study. Even so, risk management officers can feel less anxious.
Barriers in the Real World
No chemical solves everything straight out of the bottle. Price of synthesis stands tall—production of functionalized ionic liquids like [HEMIM][OAc] often costs two- to three-times more than simpler solvents. Purification chews up even more dollars. Scaling from lab to industrial vat throws in unforeseen headaches: viscosity increases, filtering becomes a pain, and recycling protocols struggle when cellulose breaks down unevenly. Still, teams at academic and industrial labs keep reporting that these ionic liquids cut down on harsh side reactions and yield higher-purity cellulose derivatives.
Pushing Innovation Instead of Playing Catch-Up
Companies and researchers can push things forward by testing blends—pairing [HEMIM][OAc] with small amounts of co-solvents to thin out the solution. Small process tweaks make a big difference. Investing in reactor redesigns and membrane-based recycling can drive prices down. Partnering with regulators can clear the fog around long-term safety and environmental profiles.
Building on my own dabbling in cellulose processing, I’ve seen that smart solvent choice makes or breaks lab budgets and sustainability goals. So, yes, 1-Hydroxyethyl-3-Methylimidazolium Acetate stands tall as a promising tool for dissolving cellulose in a world hungry for greener chemistry. Tech gaps still pepper the path, but every breakthrough here moves more than just science forward—it gives industry and the planet a shot at running cleaner.