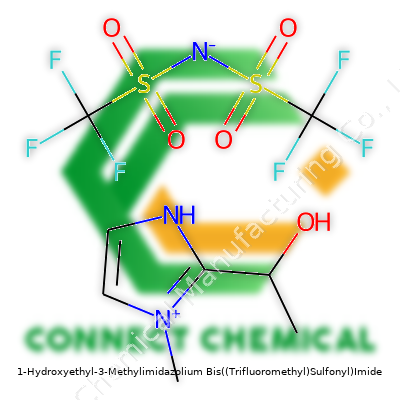
1-Hydroxyethyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: A Ground-Level Look at a Modern Ionic Liquid
Historical Development of a Modern Compound
Chemists looked for solvents that could handle tough jobs without the hazards of old-school options. In the 1990s, the field of ionic liquids took off, catching the eyes of researchers who knew that traditional organic solvents brought plenty of downsides—fire risks, volatility, environmental harm. As scientific communities dug deeper, the family of imidazolium-based ionic liquids started growing. 1-Hydroxyethyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide came on the scene as a result of this search for alternatives. Born from a blend of imidazolium cations and non-coordinating, stable anions, this ionic liquid didn’t just offer a low vapor pressure or wide thermal range; it promised consistent performance in everything from green chemistry to energy storage. Progress in synthesis, especially the handling of the tough trifluoromethylsulfonyl groups, opened the door to large-scale production and new hope for cleaner industrial processes.
Overview of the Product
This ionic liquid, sometimes labeled as [HEMIM][NTf2], comes as a clear or pale yellow liquid at room temperature. Its ease of blending into water and various organic solvents gives it a practical edge on the lab bench. Designed with a balance of hydroxyethyl and methyl groups on the imidazolium ring, the product manages both polarity and stability. Folks working with specialty chemicals look at it for more than just flexibility. They want high purity, batch consistency, and a label that’s clear enough to avoid accidents. Because this is a specialty item, every step from production to packaging leans on technical skill and tight controls.
Physical & Chemical Properties
Ask a chemist, and they'll tell you a lot rides on the physical details. The liquid state below 20°C, density near 1.4 g/cm³, and high ionic conductivity give it broad utility for electrochemistry. Hydrophilicity stands out thanks to the hydroxyethyl group, which means greater miscibility with alcohols and some water. The [NTf2] anion does more than bring fluorine atoms to the mix; it grants real chemical resilience—resistant to hydrolysis and strong acids, and barely touched by bases at room temperature. High thermal stability (often exceeding 200°C) lets users work through demanding conditions, whether in pharmaceuticals or renewable energy labs. Odor-free, nearly colorless, and slow to decompose, it’s reliable even during long experiments.
Technical Specifications & Labeling
Firms set the minimum standard at over 99% purity, keeping water content below 0.5% to avoid unpredictable reactions. Labels detail batch numbers, hazard symbols, the proper UN shipping codes for specialty chemicals, and emergency procedures for spill response or accidental ingestion. The container, usually amber glass or high-density poly, guards against UV degradation and leaching. Full documentation and CoA (Certificate of Analysis) travel with every purchase, reflecting the strict regulatory environment for laboratory and industrial chemicals. It’s not just about safety—it’s about making sure nothing slips through the cracks in a legal or research setting.
Preparation Method
Manufacturing involves the alkylation of a 1-methylimidazole precursor with ethylene oxide or an equivalent hydroxyethyl donor. This reaction, carried out in a controlled, ventilated reactor, generates 1-hydroxyethyl-3-methylimidazolium ions, forming the core of the liquid. After cation formation, the reaction shifts, introducing lithium or another alkali salt of bis(trifluoromethanesulfonyl)imide. Metathesis follows: the imidazolium halide reacts with the NTf2 salt, precipitating out the less soluble lithium halide and isolating the target ionic liquid. After separation, purification takes several distillation and drying steps—each monitored for contaminant levels, counterion impurities, and thermal decomposition side products. Without these tight routines, impurities would sabotage sensitive research or trigger regulatory headaches.
Chemical Reactions & Modifications
With the hydroxyethyl group, the cation offers a gateway for further modification, opening up possibilities for tailored catalysis or polymer-functionalization. The ionic liquid will solvate many organometallic compounds, act as a medium for transition-metal-catalyzed reactions, and even dissolve cellulose or other tough biopolymers that old-fashioned solvents can’t handle. It has a knack for stabilizing reactive intermediates, so labs use it for step-growth polymerizations and photochemical syntheses. Researchers keep experimenting—some swap methyl for ethyl on the ring, or tweak the anion to see if they get better solubility, greater electrochemical windows, or improved reaction selectivity. What stands out: the [NTf2] group rarely shifts during chemical processing, holding the backbone steady while users push the envelope with new functional groups.
Synonyms & Product Names
The market tags this compound under several names, most officially as 1-hydroxyethyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide. In catalogs, you’ll also see [HEMIM][NTf2] or its full systematic IUPAC equivalent. Other distributors might list "1-(2-Hydroxyethyl)-3-methylimidazolium bis(trifluoromethanesulfonyl)azanide." Walking the floor at a chemical expo, suppliers rattle off "hydroxyethylmethylimidazolium bistriflimide" or roll out formulae like C9H13F6N3O5S2. Behind the many names lies the same technical core—it’s consistency in the bottle that researchers care about.
Safety & Operational Standards
Since the NTf2 anion can release toxic byproducts at high heat, all procedures run with exhaust hoods and PPE—nitrile gloves, goggles, lab coats, and dedicated waste containers. Direct contact with skin or inhalation earns a call to the safety officer and likely medical evaluation. Sodium carbonate and plenty of water handle minor spills, but flammable materials stay well clear from the work zone. Material Safety Data Sheets stress the need for sealed storage, zero exposure to open flame, and the usual locked cabinet for anything that carries a mild toxicity or environmental persistence. Disposal routes through waste-management contractors, no short-cuts to drains or municipal bins.
Application Areas
Labs working on alternative energy count on this ionic liquid for its broad electrochemical window—fitting perfectly into lithium battery technology and supercapacitors. In the separation sciences, it acts as a mobile phase in liquid chromatography, providing greater selectivity for tricky analytes. Industry deploys it for cellulose dissolution, pushing into green chemistry territory that avoids volatile organic solvents. It dissolves biopolymers others can’t touch, making it a game-changer for renewable plastics and advanced textiles. Some pharmaceutical researchers trial it for soft extraction steps that leave vital biomolecules intact—no denaturation, no harsh chemicals. Electroplating and surface modification experiments rely on its chemical stability and low vapor pressure, translating to lower fire and toxicity risks. That combination of performance and safety, especially with the right protocols, keeps industry coming back for more.
Research & Development
Development teams focus on broadening the electrochemical window, lowering toxicity, and increasing recyclability. That means hunting for cation and anion tweaks, and trialing green production methods with fewer hazardous byproducts. Electrochemical studies, using both computational and bench techniques, test decomposition limits, recyclability, and compatibility with sensitive catalysts. Projects in nanotechnology leverage this ionic liquid as a matrix for producing quantum dots, nanorods, or as a template for self-assembling smart polymer systems. Open literature tracks plenty of patent activity around process improvements—everyone wants lower costs, tighter environmental controls, and wider applicability. Academic partnerships probe deep, with collaborative consortia reporting annually at major chemistry meetings. Industrial labs roll out pilot-scale methods, trying to scale the cleaner prep without losing site of purity or yield.
Toxicity Research
Studies focus on bioaccumulation and chronic toxicity, testing the fate of this compound in aquatic and soil environments. Unlike volatile organic solvents, ionic liquids don’t evaporate, so the main risk lies in accidental release to water systems or slow leaching from waste repositories. Acute toxicology tests in rats and fish track LD50 and EC50 thresholds, with most results showing moderate toxicity—that is, less harmful than halogenated organics but still a concern for industrial waste streams. Cytotoxicity runs in cell lines suggest that the hydroxyethyl group doesn’t break down into reactive metabolites, lowering long-term health risks. Environmental research monitors persistence, which tends to be slow. Proposals for enzyme-catalyzed breakdowns look promising, but need wider industry buy-in before large-scale adoption. Users push for more data, especially on chronic exposure risks for lab workers and downstream effects on aquatic biomes.
Future Prospects
Demand looks likely to keep climbing, both because sectors want greener alternatives and because old processes keep running into harder regulations worldwide. Battery technology pushes for more stable, less flammable solvents, and [HEMIM][NTf2] fits that bill. Pharma and biotech labs need extraction methods that protect sensitive materials, and this ionic liquid’s flexible polarity gives them plenty of room to maneuver. The main challenge: scaling production without bumping up costs or environmental burdens. Green synthesis methods, enzyme-catalyzed modifications, and improved recycling can set the pace. Startups and research institutes keep experimenting, driven both by regulatory changes and the need for safer lab spaces. Industry leaders, regulators, and environmental groups all look for the same thing: technical progress that walks hand-in-hand with better stewardship. How we strike that balance could set the tone for an entire field of specialty chemicals.
An Insider Look at Its Real-World Value
Scientists and engineers have been playing with 1-Hydroxyethyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide—often shortened to a complex acronym—because of its quirky abilities as an ionic liquid. This chemical doesn’t act like your average solvent. It stays liquid well below freezing, and it won’t easily burst into flames, so labs and factories see it as a supercharged alternative to old-school options.
Renewable Energy
One reason this compound matters: battery research. The buzz around electric cars and power storage always circles back to how you move ions effectively and safely. Traditional electrolytes in lithium-ion batteries catch fire or break down under heat. This ionic liquid gives batteries a higher safety margin, holds up to high temperatures and reduces the risk of ‘thermal runaway’ that people hear about when a phone or car battery explodes. Research from the Nature Energy journal shows that these ionic liquids help batteries last longer by keeping electrodes stable, holding out against chemical breakdown, and even letting cells squeeze more energy out per cycle.
Green Chemistry and Recycling
Waste from plastics and metals remains a major headache worldwide. Chemists are searching for solvents to break down plastics or extract metals from electronic waste without toxic side effects. This is where this imidazolium-based ionic liquid steps up. It dissolves tough polymers and stubborn catalysts, making it a useful tool for breaking old electronics apart and pulling out precious metals. I’ve talked with engineers who prefer these ionic liquids for their lower volatility—they don’t stink up the lab or damage equipment from fumes, and they recover more of what’s valuable from trash that would otherwise wind up in landfills. Data backs it up: extraction yields can jump substantially when swapping out classic chemicals for one like this.
Chemical Manufacturing and Synthesis
Lab workers love reliable reactions. This ionic liquid steadies chemical reactions that traditional solvents can’t manage, and it doesn’t dry up or degrade so easily. Pharmaceutical companies use it for specialized reactions where water or other solvents ruin yields. Fine chemical makers trust it for synthesizing molecules that fall apart if the chemistry isn’t perfect. Publications in ACS Chemistry of Materials highlight how these ionic liquids improve purity in drug and polymer production, which means less waste and fewer headaches later on.
Potential Drawbacks and Solutions
Not everything about this ionic liquid sparkles. Cost hits hard, especially at scale. Production can take a toll on the environment due to fluorinated building blocks, which stick around in soil and water. Some research teams have flagged toxicity risks to aquatic life if it escapes into groundwater. Regulations like REACH in the EU continue tightening screws on persistent chemicals. To tackle these problems, teams keep experimenting with less persistent or biodegradable versions and recycle used ionic liquid as much as possible. Industry circles now push for full life-cycle assessments—tracking every batch from start to finish—to shrink the footprint and protect ecosystems. In my view, transparency about chemical origins, smart recycling, and collaboration between chemical suppliers, recyclers, and environmental scientists could curb the biggest downsides.
The world looks to solutions that balance performance with responsibility. This ionic liquid proves progress doesn’t have to settle for unsafe or old-fashioned options. We can push materials science ahead and still watch out for our planet.
The Reality of Ionic Liquid Storage
Walking through any research lab, it’s easy to spot the growing collection of ionic liquids lining the shelves. Despite the excitement over their versatility, I’ve noticed questions about just how stable these bottles stay over time. Every scientist who’s cracked open a sample after six months knows the sinking feeling of finding a yellowed liquid, or one that smells a bit off. The label might promise “stable under standard conditions”, but there’s room for disappointment if you trust that line without second thought.
Hands-On Experience Shows the Cracks
Years working in chemical development taught me to treat every bottle as a living thing, especially with ionic liquids. The textbook might describe them as “thermally stable” or “non-volatile”, yet reality doesn’t always match the literature. I’ve run into issues with even the top-shelf imidazolium salts, which can oxidize in glass under air unless you keep them dry and away from sunlight. In one project, we lost three weeks of work because our ionic liquid, supposedly safe at room temperature, had turned acidic from trace moisture.
Realistically, labeling conditions as “standard” leads to a lot of trouble. What’s standard in one lab might not look the same in another. In Australian summer, room temperature isn’t always 22°C, and humidity sneaks in even with tightly screwed lids. Even specialty storage cabinets can’t fix private lab habits, when students often forget to flush bottles with nitrogen after use.
Science Unpacks the Details
Data on ionic liquid stability keeps piling up. For example, fluorinated ionic liquids can break down and produce highly toxic products like HF if exposed to water for too long. The classic [BMIM][BF4] suffers in open air, absorbing water and sometimes hydrolyzing the anion. Often, manufacturers supply a product pure out of the bottle, but the actual material starts degrading the moment you open it, especially if it pulls moisture or CO2 from the air.
A study from the University of Bremen (J. Chem. Eng. Data, 2014) tracked the hydrolysis of ionic liquids under ambient humidity, watching as acid byproducts formed quietly over weeks. Even the most inert-seeming samples, like sulfonium salts, can show discoloration and lose unique properties. Despite claims of “wide liquid range” or “chemical robustness”, the practical side tells a different story.
Solutions Lived, Not Just Theorized
Rather than blaming the chemicals, a lot comes down to how they’re handled and stored. In my own workflow, I turn to amber glass bottles to keep light from catalyzing slow breakdown. Storing under inert gas made huge improvements for anything with sensitive anions. Desiccators might take up precious space, but I still recommend them for labs without climate control. Repacking ionic liquids after each use, or dividing supplies into smaller vials, can keep a whole batch from degrading at once.
Education also matters. Anyone working with ionic liquids should get the real scoop, not just the rosy details from catalogues. Encourage researchers to check for color changes, off-odors, or shifts in viscosity before throwing a sample into a reaction. These signs often tell the truth faster than a purity assay.
Building Trust Through Transparency
Trust only builds when both producers and users stay open about the real performance of ionic liquids. Chemical catalogues must stop leaning on “standard conditions” as a catchall. Share shelf-life data, teach about risks, and make better packaging standard. Researchers need to talk openly about failures, not just successes, in literature and at conferences.
Stability isn’t just a property someone prints on a datasheet. It’s a combination of good manufacturing, honest storage, and careful daily habits in the lab. Staying grounded saves time, money, and heads off a lot of headaches in the end.
Looking Closer at Purity
Every bottle or shipment of a chemical represents more than just raw material. Its value rises or falls on purity. For labs, factories, and healthcare settings, this single detail changes everything — from safety to product quality. People often think a chemical is just "what it says on the label," but that idea misses a critical fact: tiny amounts of other substances often sneak in.
I’ve spent years seeing first-hand what happens if labs cut corners or skip clear specs. Impurities introduce risks. Sometimes, they cause equipment to clog or research to give false signals. In medicine or food, a batch filled with contaminants might mean real harm. It rarely gets discussed outside the industry, but pure compounds are foundational for clean results, safe outcomes, and fair competition.
Understanding the Assay
Assay specification isn’t some confusing jargon. It’s a simple concept: a precise measurement of active ingredient in a given sample. Good suppliers clearly state this—usually as a percentage.
Take sodium chloride for example. In most pharmaceutical or laboratory use, you'll see something like “≥99.5%.” This number shows just how much of the substance truly matches the label. Spotty reporting, with vague or missing assay numbers, should send up a red flag. That means no one double-checked or stood behind their product.
Without strict assays, manufacturers risk real trouble. Performance drops. Bad batches slip into circulation, hurting both trust and business. Investigations into contaminated products always highlight missing or ignored purity specs.
Why strict specs protect everyone
Most chemical customers ask about ‘purity’ early in the buying process. They’ve been burned before or know colleagues who have. Too often, someone receives material that looks fine but holds enough water, ash, or metals to disrupt a process or even pose direct hazards. Companies using clear specifications, certificates of analysis, and third-party verification wind up ahead.
I recall one supplier switching venders to cut costs. The quieter, cheaper source didn’t provide certificates showing batch-to-batch quality. Within months, failed reactions and lost batches hit the company hard. Only careful tracking of purity and assay would have stopped that loss.
Setting Better Standards
What really helps is a culture of transparency and communication along the supply chain. Reputable producers always publish detailed specs—exact percentages, lists of allowed contaminants, and test methods. Regulatory standards help here. The USP (United States Pharmacopeia), EP (European Pharmacopeia), and food compendia list concrete specs for many substances. Companies sticking to them avoid recalls and courtrooms.
No one wants their next delivery of a raw ingredient to be a source of trouble. Requesting purity and assay information, demanding documentation, and running spot checks send a clear message. Every batch must meet set targets. Science, health, and quality control never thrive on guesswork or hidden surprises.
Respect for Safety Starts Before the First Step
Getting to know a chemical starts with respect. I remember my first experience in a university lab, staring at a bottle labeled with a string of warnings. One quick glance told me that casual mistakes have real consequences. People often think a set of gloves will protect them from anything, but that’s far from true for most substances on a chemist’s shelf.
The Hidden Dangers: Don’t Underestimate the Risks
Chemical bottles can look harmless, but many contain powders or liquids that can irritate skin, damage eyes, or harm your lungs. Some compounds, once spilled on a bench, never come out, and years later, still carry faint warnings. Others, like concentrated acids or bases, cause burns with just one drop. Familiar names like acetone and bleach can quickly turn dangerous without ventilation, since breathing their vapors for long periods damages more than just your nose — the effects stretch to the lungs and, sometimes, the brain.
Expert Advice is the Best Defense
People with years of laboratory experience never skip reading a safety data sheet (SDS). That habit protects not just themselves, but everyone nearby. Key facts include the correct gloves — nitrile resists many solvents, but not all. Splash goggles protect against a careless hand knocking over a beaker. Lab coats and closed shoes prevent accidents from sending someone to the emergency room.
Storing It Right is Half the Battle
Most incidents happen because someone stores a compound near something incompatible. Acids kept near bases often lead to leaks and clouds of noxious gas. Flammable substances, if exposed to a spark or stored by a heat source, can ignite and spread quickly. A cluttered shelf makes mixing up bottles all too easy. Labels fade or fall off, turning yesterday’s careful organization into today’s guessing game. I saw a student once mistake an oxidizer for table salt, leading to a near-disaster that pushed home the message: storage instructions aren’t just theoretical.
Proper Disposal Keeps Hazards From Lingering
Tossing chemicals down the drain is not just bad for the plumbing — it ruins water supplies and endangers wildlife. Dangerous waste needs its own bin, collected and dealt with by trained teams. Treating waste with the same care used during the experiment keeps harmful compounds out of rivers and food chains, and local communities remain safe.
Looking Forward: Training and Honesty Matter Most
Good habits don’t come from reading rules on a dusty poster. Real improvement grows out of honest conversations about accidents and close calls. People learn from each other, swapping stories of things gone wrong so rookies don’t repeat them. Open reporting plays a part — it connects small mistakes to better practices, shaping safer workplaces across labs and factories.
Simple respect for chemical hazards remains the strongest tool we have. Gloves, goggles, and storage protocols shape a place where people look out for each other and science gets done without tragedy. In every workplace, from universities to pharmaceutical companies, that sense of responsibility makes all the difference.
Real-World Demands Go Beyond Off-the-Shelf Options
Every lab worker and production engineer has run into this: a catalog product almost fits the project, but the “almost” turns into days spent patching solutions together. Off-the-shelf isn’t always enough. I’ve watched research teams waste valuable hours tinkering with supplies, only to bump against built-in limits. These issues don’t just slow things down—they drive up costs. According to the National Institute for Innovation in Manufacturing, companies that rely too much on standard stock spend roughly 30% more adjusting processes than groups who source or build tailored components.
Why Flexibility Matters on the Ground
One-size-fits-all rarely delivers. A polymer chemist searching for a reagent stable at high pH, or a food scientist testing a high-protein ingredient for new snacks, finds that tiny tweaks can make or break experiments. In my graduate years, I learned quickly that compromising on test materials often made results unpredictable. Only shifting the base properties—particle size, solubility, purity grade—led to breakthroughs. This hands-on reality shows up in industry as well. Automotive coatings, for example, need custom pigment blends to beat harsh weather, or sensors in medical diagnostics require specific surface treatments for fast and reliable detection.
Barriers to Customization in the Real World
Getting something changed for a project seems easy—until the cost and logistics roll in. Smaller firms, or academic labs, often face steep minimum orders or long delivery times. Big producers hesitate to switch gears for what might be a single batch. In some cases, regulations add extra hoops for non-standard goods. It’s a pain point rarely addressed by glossy catalogs. I’ve known companies forced to sit out entire bids because suppliers couldn’t accommodate custom options within deadlines. The loss isn’t just money, but also missed chances to innovate or enter new markets.
What Builds Trust and Results: Conversation and Know-How
Customization works best with open conversations. In my own projects, only after sitting down with a supplier’s technical team did the right questions surface. Are heavy metals below trace thresholds? Does the formulation run on existing equipment? Good vendors ask about the problem, not just the specs. They rely on certified data—purity tests, stress testing—and will walk through the documentation. Anyone can say “yes, we can change that,” but results depend on transparent dialogue and clear track records. Google’s E-E-A-T framework highlights this—trustworthy products come from transparent origins and shared expertise.
Solutions that Close the Gap
Several paths open doors for those needing custom products. Collaborative platforms now link buyers directly to technical staff from producers, bypassing generic sales pitches. Modular production lines help manufacturers respond faster with smaller runs. For regulated sectors like pharma or food, some firms keep “blank slate” lots—verified raw materials that can be finished to custom specs and shipped after a final round of tests. These approaches speed up delivery and allow more precise adaptation, often cutting lead times by weeks.
Moving Forward: A Culture Shift
Demand for customization shows no sign of slowing. More companies realize that building the right solution from the ground up supports cleaner data, more efficient factories, and safer products. It’s not just about chemistry or hardware—success depends on building genuine partnerships grounded in proven expertise and consistent results.