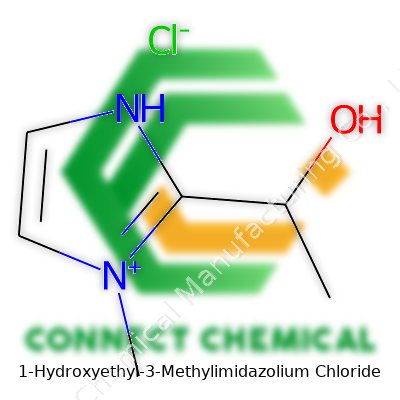
1-Hydroxyethyl-3-Methylimidazolium Chloride: A Down-to-Earth Dive Into a Versatile Chemical
Historical Development
Chemistry rarely stands still, especially when folks keep searching for greener, flexible solutions in solvents and reaction media. Work on imidazolium salts took off once researchers craved alternatives to volatile organic compounds. By the 1980s and 1990s, people noticed these salts didn’t just dissolve tough stuff—they stayed liquid under common conditions. The birth of 1-Hydroxyethyl-3-Methylimidazolium Chloride answered practical calls for ionic liquids that mix well with both water and organic molecules. One thing becomes clear from looking back: every small tweak on the imidazolium ring pushed science a tad closer to safer labs, better recycling, and smarter reactions.
Product Overview
1-Hydroxyethyl-3-Methylimidazolium Chloride stands out as a classic example of an ionic liquid thriving well beyond novelty status. It brings together the hydroxyethyl and methylimidazolium groups with a chloride counterion, making a substance that pours easy, looks either as a colorless or pale yellow liquid, and resists evaporation even during heavy use. Labs and industry like it because it dissolves a wide range of things—salts, organic compounds, probably even some stubborn stains from the lunchroom microwave.
Physical & Chemical Properties
You pick up a bottle of this chemical, and you notice its mild viscosity—nowhere near syrup, but not quite water. Its melting point usually stays below 100°C, sometimes even liquid at room temperature. Solubility in water comes easily, letting scientists blend it into reactions with little trouble. The hydroxyethyl side allows some hydrogen bonding, which shifts how it interacts with other molecules compared to “plain” imidazolium salts. It won’t burn up like alcohol or acetone; it does not catch fire quickly, which is good news on hectic days. It withstands a broad range of pH, managing to remain intact unless someone tries really harsh acids or bases.
Technical Specifications & Labeling
Manufacturers label this salt by purity, water content, and color. A reputable bottle will note imidazolium chloride percentage, and levels above 98% show up in most high-grade labs. Spec sheets often mention refractive index, density, and levels of residue after evaporation. For regulatory compliance, proper hazard pictograms and UN shipping codes accompany commercial shipments. Labeling not only keeps the safety officer happy, it helps researchers avoid wasted time and botched results due to impure stock.
Preparation Method
Industrial chemists prepare this molecule by quaternizing 1-methylimidazole with a haloalcohol like 2-chloroethanol, followed by reaction with hydrochloric acid. Purification matters, so repeated solvent washes and careful evaporation remove unwanted by-products and leave the final salt dry and ready to use. Scaling up for commercial delivery requires good control of reaction temperature, timing, and reactant ratios, or else the yield and purity take a hit. People in the business swear by precise batch monitoring and consistent starting material, since slack leads to surprises at scale.
Chemical Reactions & Modifications
This molecule doesn’t just sit around as a solvent. It can swap its chloride ion for other anions, turning into ionic liquids with new properties. Chemists attach new groups to the hydroxyethyl side, sometimes to add reactivity or to control how it interacts with metals and catalysts. The imidazolium ring itself gets tweaked as researchers chase stronger conductivity, better solvation, or milder toxicity. A knack for these modifications keeps the field lively and helps the salt break out of single-use territory.
Synonyms & Product Names
People might call this compound 1-(2-Hydroxyethyl)-3-methylimidazolium chloride, or [HEMIM]Cl in shorthand. Trade names pop up depending on the vendor; some catalogs just list it under its CAS number or roll out abbreviated forms. No matter the name, it’s recognizable in the growing ionic liquids market.
Safety & Operational Standards
Using 1-Hydroxyethyl-3-Methylimidazolium Chloride, like most chemicals, requires common sense and solid standards. Always wear gloves and splash goggles during handling to avoid skin or eye irritation. Despite its low volatility and general stability, spills need immediate cleanup since dense ionic liquids create slick surfaces. Long-term storage works best in tightly sealed bottles, away from strong acids or bases, to prevent hydrolysis or slow decomposition. Modern labs monitor air handling closely, because even “green” solvents sometimes build up fumes after repeated use. Universities and companies now demand comprehensive safety training, which makes working with these newer materials far less risky than it used to be. They care about employee health, regulatory fines, and lab reputation—neglecting any of these leads to trouble.
Application Area
Ionic liquids like this hydroxyethyl derivative become part of separation technologies, catalysis, and even in stabilizing enzymes for greener chemical processes. Some teams run organic syntheses using this salt as a replacement for traditional flammable solvents. People in battery research test it for safer electrolytes, and polymer manufacturers use it to dissolve tough plastics or create stable coatings. No matter the field, adaptability and environmental performance draw attention. As companies try to cut back on waste and toxic by-products, demand for versatile, lower-impact chemicals grows fast.
Research & Development
Academics across continents spend resources on ionic liquids. The challenge centers on combining strong performance with lower toxicity and easier recycling. The hydroxyethyl group offers intriguing avenues for further fine-tuning interactions with metals, organics, or biological materials. Grants go to predictive modeling and combinatorial approaches so teams don’t just test thousands of random compounds. My own experience working with research teams taught me that bench work alone no longer suffices; interdisciplinary groups move fastest, with chemists, engineers, and environmental scientists throwing new ideas into the mix.
Toxicity Research
No chemical escapes the eye of toxicologists, and 1-Hydroxyethyl-3-Methylimidazolium Chloride gets no pass. Testing in labs shows that—compared to older solvents—ionic liquids tend to have lower vapor toxicity, which benefits air quality in closed rooms. But just because it doesn’t evaporate fast doesn’t make it inert; aquatic toxicity remains a concern. Studies document that fish and aquatic invertebrates can react to low concentrations, especially if labs or factories do not filter waste streams properly. Chronic exposure data remain spotty in some areas; industry can do more to fund transparent, long-term tests. Researchers want clear answers on breakdown products, bioaccumulation, and disposal protocols. Luckily, regulatory pressure and journal demands push teams to publish both positive and negative results these days.
Future Prospects
Economies shift toward cleaner materials and processes, which puts chemicals like 1-Hydroxyethyl-3-Methylimidazolium Chloride in the spotlight. Demand for safer solvents and functionalized materials isn’t just a fad; it grows as industries answer consumer pressure and regulatory demands. My bet is that, over the next decade, companies invest more in designing analogues with even lower toxicity, tighter life-cycle controls, and built-in recycling protocols. Chemistry graduates reading this today could end up working on these challenges in a few years, pushing boundaries while balancing cost, ethics, and performance. This compound, in all its synonyms and slightly altered versions, has a front-row spot in tomorrow’s cleaner, more efficient lab—and a solid place in my own chemical tool kit.
Understanding the Compound
1-Hydroxyethyl-3-methylimidazolium chloride belongs to a class of chemicals called ionic liquids. These liquids behave quite differently from the usual solvents you find in labs or factories. Their low volatility and strong thermal stability mean they don’t evaporate easily or break down at high temperatures. The structure of this particular ionic liquid—an imidazolium ring with a hydroxyethyl and a methyl group attached—gives it special properties that chemists love to work with.
Real-World Uses: From Lab Benches to Industry
Most people don’t come across ionic liquids in their day-to-day life, but that doesn’t mean they matter less. This compound’s main work shows up in the world of chemistry and materials science. Many researchers turn to 1-hydroxyethyl-3-methylimidazolium chloride for its usefulness as a solvent. It can dissolve a variety of organic and inorganic compounds—something water or traditional organic solvents struggle to do. One detail I’ve noticed in research circles is the push for greener processes. This ionic liquid helps in that direction, since it doesn’t release smelly vapors or hazardous byproducts into the air.
Helping Chemistry Move Forward
One place I kept seeing this compound pop up was in cellulose processing. Take the common tree: turning wood pulp into usable fibers or films often needs an aggressive cocktail of chemicals. This specific ionic liquid dissolves cellulose efficiently and lets experts spin it into fibers or cast it into transparent films without the heavy environmental penalty. A research paper from Green Chemistry highlights how replacing old solvents with ionic liquids brings real gains in sustainability.
Beyond cellulose, this compound also proves itself in catalysis. In catalytic processes, the right solvent influences how fast and cleanly a reaction moves. Scientists use 1-hydroxyethyl-3-methylimidazolium chloride to speed up reactions, improve yields, and cut down on chemical waste. It also handles metal extraction and recovery—say, pulling valuable metals out of electronic waste—without creating a stew of hazardous leftovers.
Energy Storage and Electronics
This ionic liquid extends into the world of energy. I’ve read papers pointing to its use in electrolytes for batteries and capacitors. Because this liquid doesn’t easily catch fire or boil away, it helps boost the safety and lifespan of lithium-ion batteries, which run everything from laptops to electric cars. Lab tests show more stable cycling in batteries using ionic liquids, compared with traditional organic electrolytes.
Looking at the Bigger Picture: Challenges and Solutions
One honest concern with ionic liquids has always been their cost and environmental life cycle. While these compounds help reduce emissions and hazardous waste during use, making them eats up energy and raw materials. More research is focusing on how to recycle or reuse these liquids to cut the total impact. Some labs reclaim ionic liquids after processing and purify them for further cycles, lowering both cost and environmental burden. Clearer guidelines about toxicity and biodegradability could help, too, since all new chemistry brings risks as well as rewards.
In the end, 1-hydroxyethyl-3-methylimidazolium chloride stands out not just for what it does in a flask, but for its role in pushing chemical and industrial techniques away from unsustainable practices. The chemistry community keeps asking tough questions about safety and sustainability, and this ionic liquid—when used thoughtfully—lets them shape better answers.
Getting to Know the Chemical
1-Hydroxyethyl-3-Methylimidazolium Chloride hasn’t broken through to household-name status, though folks in labs and industrial spaces may recognize this ionic liquid. Researchers and manufacturers value it for its role in dissolving cellulose, facilitating organic synthesis, and other specialized uses. That all sounds well and good, but stable storage often gets overlooked. Skipping those basics is like leaving milk on the counter: It spoils before anyone can use it.
Practical Storage for Long-Term Stability
I’ve seen enough lab surprises to know: Poor storage invites unpredictable outcomes. This chemical stays happiest at room temperature, away from open air and sources of moisture. Why? 1-Hydroxyethyl-3-Methylimidazolium Chloride loves to suck up water. If humidity creeps in, purity slips and performance drops. Leaving the cap loose seems minor, but water in the atmosphere finds its way in. That shift can throw off reactions, clump stored powder, and generally mess with data quality.
Every solid has natural enemies. For this ionic liquid, sunlight doesn’t do any favors. UV rays may trigger degradation, causing yellowing, loss of function, or unwanted side products. Opaque containers or those amber glass bottles you see in labs do more than look nice—they block out light and reduce risk.
Why Contamination Drags Everything Down
No one sets out to spoil a batch by accident. Contamination happens quietly. Even moving a spatula from one bottle to another poses a risk. Keep this compound in a dedicated, tightly sealed container, marked clearly to avoid mix-ups. Silica gel packs go a long way in fighting stray moisture; so does a thoughtfully placed desiccator cabinet. Reliable labeling and diligent log-keeping might sound tedious, but it stops a lot of waste. Once, I watched a colleague chase down a mystery impurity for a week—only to trace it to sloppy storage. A five-second twist of the lid would have saved time and money.
Health, Safety, and Legal Responsibility
Oversights risk more than bad data. Ionic liquids sometimes raise health concerns for skin and eyes, so avoiding leaks or spills matters. Good technique means wearing gloves, goggles, and keeping workspaces clear. Local regulations require chemical storage that keeps hazardous substances secure and labeled. Violations invite more than fines; they put people at risk, which is never worth the shortcut.
Battling Common Storage Issues
Maybe a supply closet lacks climate control or a fume hood sits near a sunny window. Storage upgrades don’t need to cost a fortune. Simple tactics—switching to airtight containers, relocating to a shaded shelf, or introducing silica packets—pay off quickly. For big batches or long-term storage, a purpose-built chemical refrigerator may help. Always check safety guidance from suppliers and follow local codes.
Consistency Protects Quality and Cost
Good storage habits shape reliable results and stretch budgets. Every careless exposure means quality drops or a reorder pops up on the purchasing list. Getting this right shows commitment to safety, science, and the well-being of everyone in the building.
Putting in the extra bit of effort on the front end—tight lids, dry surroundings, a little darkness—keeps 1-Hydroxyethyl-3-Methylimidazolium Chloride ready for the next big experiment or project.
Understanding the Chemical
1-Hydroxyethyl-3-Methylimidazolium Chloride, a mouthful even for folks with science backgrounds, shows up as a kind of ionic liquid. These chemicals pull in a lot of attention because of what they can do in labs and factories. Many companies use ionic liquids for things like breaking down cellulose, helping with chemical reactions, and even pulling out metals from waste. While these applications sound promising, safety deserves a bigger spotlight.
Weighing Toxicity Risks
To figure out risks, facts matter. For this compound, official, peer-reviewed studies remain limited compared to older, more widely used chemicals. Studies on the broader class—imidazolium-based ionic liquids—suggest skin and eye irritation can occur if you’re careless. High doses might trigger other problems such as cytotoxic effects, meaning they can damage living cells. Researchers found in some tests that these substances impact aquatic life, with fish and crustaceans suffering from even low levels.
Long-term health information for workers directly exposed still looks thin. Most safety data sheets urge avoiding contact and breathing in dust or mist. They tend not to label it immediately deadly, but absence of strong proof shouldn’t mean we turn our backs on caution. Standard laboratory practice means gloves, goggles, and lab coats—these aren’t for show. Even small spills should be cleaned up right away to stop slips and unplanned skin contact. Chemical fume hoods cut down on inhalation risks. Relying on best practices goes a long way in keeping people safer where data leaves off.
Environmental Considerations
Manufacturers sometimes pitch ionic liquids as green because they barely evaporate and often replace flammable solvents. Green credentials fall apart if residues harm water supplies or wildlife. After use, improper disposal can let these chemicals slip through treatment plants since standard filters don’t always catch everything. In the environment, some ionic liquids stay put instead of breaking down, and persistence like this spells trouble in rivers and soil. Even minuscule amounts can disrupt growth in algae and small aquatic creatures, which anchor the food chain. If farm irrigation or drinking water picks up traces, bigger issues could surface for both animals and humans over time.
What Responsible Use Looks Like
The solution starts with staying informed. Before bringing 1-Hydroxyethyl-3-Methylimidazolium Chloride into a classroom, lab, or plant, workers should receive real-world safety training. Managers must give employees the right protective gear and keep up with updates in chemical safety literature. Engineers and scientists can dig into newer research that looks at long-term health effects, not just quick tests on mice or plants. Any waste should go into specialized containers and move to hazardous-waste processors familiar with tricky chemicals. Care in labeling and storage matters too, because confusion between similarly named chemicals risks avoidable mistakes. Better research will keep filling in the gaps about chronic exposure and low-dose effects, but until that knowledge arrives, prudence beats regret every time.
Supporting Claims with Facts
The European Chemicals Agency lists some imidazolium ionic liquids as hazardous, based on consistent data about irritation and aquatic toxicity. In real-life cases, workplaces that bought into the “green” marketing without proper handling suffered chemical exposure incidents and had to pay out fines or compensation. Responsible labs adopt the Precautionary Principle, meaning they treat unknown risks as real until proven otherwise. Local regulations may require regular health monitoring or limit the total amount stored on site, a rule based on lessons from toxic spills in the past. People sometimes ignore chemical labels because the names feel foreign and the hazards seem abstract, but direct consequences have already happened in communities around the world when substances labeled as “likely safe” turned out otherwise.
Understanding What We’re Dealing With
Working with chemicals day in, day out, brings a real respect for what goes on in the lab. 1-Hydroxyethyl-3-methylimidazolium chloride belongs to the group of ionic liquids scientists use for all kinds of research, especially because it dissolves a wide range of substances. That power can be a blessing—or a headache—if you aren’t careful. At the bench, safety is only ever as strong as your next move, and this compound deserves your full respect. I’ve seen enough close calls in academic settings to know small shortcuts lead to big regrets.
The Gear You Need
Nobody should approach this kind of chemical without personal protective equipment. In my experience, gloves—preferably nitrile, as latex sometimes gets degraded—are step one. Chemists never forget eye protection: goggles with side shields give you real peace of mind when pipetting liquids that can splash. If you’ve ever gotten a splash in your face, even from water, you know the shock that comes with it. Add a lab coat and keep your work area organized to reel in unintentional exposures that can come from clutter and distraction.
Ventilation Matters
Despite claims about ionic liquids being “green” solvents, I don’t care for that buzzword when it leads to carelessness. Some ionic liquids can give off hazardous fumes, especially if heated. Moving procedures into a fume hood stands out as the smartest move—not only does it keep vapors out of your lungs, but it also assures that if something does spill, you won’t have lingering odors or airborne chemicals to worry about. Decades of research point to chronic exposure risks even for seemingly mild vapors, so it’s wise to limit every chance you get.
Spill Response: Learning from Experience
No one expects to spill a beaker—but everyone eventually does. If the spill lands on metal, the reaction can sometimes be unpredictable; I’ve seen rust stains and strange bubbling where organic salts hit older lab benches. Grab absorbent material and scoop up the liquid, avoiding paper towels that shred and spread the mess. Dispose of waste right away, following your chemical safety protocol. Soap and plenty of water clean up the last bits on your skin if you make contact. No one should take chances with questionable cleanups; I once witnessed a rushed job lead to slippery floors and the next morning’s accident.
Storage and Labeling
Too many labs tuck bottles of specialty chemicals away in crowded cabinets. I recommend giving 1-hydroxyethyl-3-methylimidazolium chloride its own spot, away from acids or bases. I label every container with the full chemical name, hazard symbols, and preparation date. In an emergency, having clear labels stops colleagues from guessing. Before locking up for the night, check lids, wipe bottles, and make mental notes of any leaks. Chemical safety disasters often follow lapses in attention to these basic steps.
One Eye on Disposal
Ionic liquids challenged disposal routines since they're less volatile but not always less toxic. Don’t dump used solutions down the sink. Follow your institution’s protocol—most will have specialty chemical waste containers. Share any uncertainty with your EHS team. I keep a digital folder with SDSs for quick reference and encourage everyone in the lab to do the same. Good habits outlast even the most convenient shortcuts.
Understanding the Basics
The name 1-Hydroxyethyl-3-Methylimidazolium Chloride may sound intimidating to folks who don’t have a chemistry background, but the core facts are simple. This compound belongs to a family of ionic liquids that researchers and industrial labs rely on for their unique properties. Its chemical formula: C6H11ClN2O captures all the elements involved—six carbon atoms, eleven hydrogens, one chlorine, two nitrogens, and one oxygen. For people working in labs or manufacturing, getting this formula right means the difference between success and frustration.
Why Purity Stands Out
High purity is not just a nice-to-have. In applications like pharmaceuticals, bio-catalysis, or advanced materials, even a trace of contamination can throw off an entire process. I'd compare it to baking: even a pinch too much salt changes the whole cake. For this compound, most manufacturers report purity above 97%, and some go as high as 99%. Reaching this level involves a careful process of synthesis and repeated purification, often using vacuum drying and sophisticated filtration. Labs usually check the product with NMR spectroscopy or high-precision chromatography, because relying only on supplier labels can lead to costly errors.
The Real-World Stakes
Some years back, I worked beside a team engineering polymer electrolyte membranes. They ran into real trouble with a batch of 1-Hydroxyethyl-3-Methylimidazolium Chloride labeled as “98% pure.” The remaining 2%—which the supplier did not detail—contained water and an unknown organic byproduct. After weeks of mysterious inconsistencies, we traced the problem to that impurity. Repeated production runs failed, budgets swelled, and morale tanked until the team sourced a new, verified supply. That opened my eyes to the personally felt costs of impurity in research and industry. Clean inputs mean smooth progress. Even outside technical fields, almost everyone has tasted the frustration of “good enough” materials turning out to be not quite good enough.
Solutions and Paths Forward
Verifying purity does not come down to buyer’s luck or trusting certificates alone. Smart labs sample new lots for testing before critical experiments. Some companies insist on third-party analysis rather than relying on a supplier’s numbers. In my experience, collaborating directly with suppliers pays off; a little transparency about production methods helps avoid surprises. Investing in better purity up-front may seem expensive, but repeated failures and rework always cost more.
Looking Beyond the Formula
It’s tempting to focus only on the formula—C6H11ClN2O—without thinking about what might hitch a ride along with the purchased chemical. As research gets more complex and products become more sensitive, both purity and transparency become crucial for progress. For anyone in a lab setting, the lesson looks clear: know your chemicals not just by name or formula, but by the details only rigorous testing reveals. If you’re putting faith in a supplier, it pays to ask questions, run your own checks, and treat every batch as a unique case.
References
- National Center for Biotechnology Information. PubChem Compound Summary for CID 44138208, 1-Hydroxyethyl-3-methylimidazolium chloride.
- J. Chem. Eng. Data 2016, 61, 485–492. “Physical and Chemical Data for Ionic Liquids.”
- Supplier information and real-world QA protocols from laboratory practice.