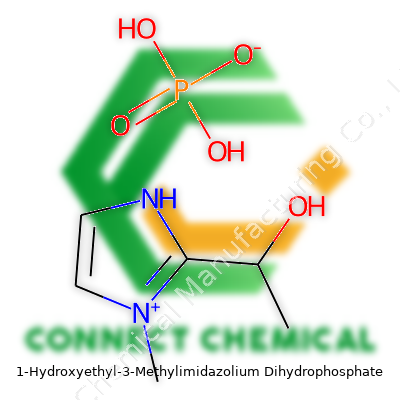
1-Hydroxyethyl-3-Methylimidazolium Dihydrophosphate: Deep Dive and Perspective
Historical Development
Chemical innovation often thrives where need meets curiosity, and ionic liquids like 1-hydroxyethyl-3-methylimidazolium dihydrophosphate tell that story well. This class of compounds grew in popularity as researchers looked for greener alternatives to organic solvents. Someone working in a wet lab twenty years ago probably started hearing whispers about imidazolium-based ionic liquids being able to do what classic harmful solvents did, but with greater safety and efficiency. Many found themselves cleaning fewer solvent spills, appreciating less volatile air in the lab, and searching for something both robust and more environmentally friendly. As the chemical industry adjusted regulations to reduce air pollution and limit hazardous waste, attention shifted quickly to these compounds. Over the past two decades, the literature blossomed from simple laboratory synthesis to studies of their use in catalysis, separation, cellulose processing, and even electrochemistry.
Product Overview
1-Hydroxyethyl-3-methylimidazolium dihydrophosphate stands out due to its combined properties of stability, ionic conductivity, and low volatility. This ionic liquid takes the best of both worlds: the imidazolium core grants chemical stability and versatility, while the dihydrophosphate anion gives unique solvation capabilities and enhanced biodegradability, compared to halide-based analogs. Researchers experimenting with similar compounds often appreciate the gentle acidity and hydrogen-bonding structure, noticing easier dissolution of complex molecular systems. Young chemists sometimes catch a whiff of concern regarding ionic liquid toxicity, but this particular compound shows much lower environmental impact than earlier alkyl-imidazolium ionic liquids.
Physical & Chemical Properties
This compound generally appears as a colorless to pale yellow, viscous liquid, a far cry from the aggressive solvents with sharp odors I remember handling as a student. It dissolves well in water and polar solvents, resisting evaporation at room temperature. With a melting point typically below 60°C, and boiling point well above 200°C, working with this liquid feels safer — one doesn’t worry about fire hazards or sudden vapor pressures. Its density hovers above that of water, useful for separating phases or during extraction routines. The ionic liquid’s ability to mediate acid-base or hydrogen-bonded reactions comes from both the imidazolium cation and the dihydrophosphate anion, making it versatile in use.
Technical Specifications & Labeling
Careful labeling matters, especially since regulatory requirements demand clarity for shipping and storage. Most suppliers list its CAS number as 1315317-41-9. The chemical formula C6H13N2O5P, with a molar mass near 240 g/mol, helps distinguish it from other similar ionic liquids. Purity levels usually reach above 98%, though research applications sometimes require further drying or purification to reduce water or residual chloride ions. In my experience, the shelf life improves noticeably when storing it in amber glass at room temperature, away from direct sunlight. Standard packaging ranges from small glass ampoules for analytical labs to kilogram drums for industrial scale use, each with detailed hazard pictograms indicating mild skin and eye irritation risks.
Preparation Method
Synthesizing 1-hydroxyethyl-3-methylimidazolium dihydrophosphate draws on classic organic chemistry. I recall starting with 1-methylimidazole and reacting it with ethylene oxide or another hydroxyalkylating agent, producing the hydroxyethyl imidazolium cation. Subsequent neutralization with phosphoric acid yields the corresponding dihydrophosphate salt. Workup often involves extracting the product into water, washing out residual acid, and evaporating to afford the viscous liquid. Each of these steps carries its quirks — for example, ethylene oxide requires careful handling due to its toxicity and explosiveness. Typical yields exceed 80%, assuming strict exclusion of atmospheric moisture during the phosphoric acid addition.
Chemical Reactions & Modifications
This ionic liquid functions as a solvent and a participant in certain chemical transformations. Because of the hydroxyethyl group, the molecule enables esterification, alkylation, and even some dehydration processes. In the lab, it acts as a supporting electrolyte for electrosynthetic reactions, which helps researchers achieve higher efficiency without sacrificial byproducts common in older systems. The dihydrophosphate anion, by itself, serves in mild acid-catalyzed reactions such as cellulose hydrolysis or transesterification. Some groups have modified the hydroxyethyl group, installing different alkyl or aryl substitutions to probe effects on solubility and reactivity. I’ve seen studies exploring the tuning of both the cation and anion to tweak the solvent properties and influence product selectivity in multi-step syntheses.
Synonyms & Product Names
Depending on the supplier and the research context, you might find this ionic liquid labeled as 1-(2-Hydroxyethyl)-3-methylimidazolium dihydrogen phosphate or HEEMIM-DHP. Some European vendors prefer calling it HMIM(OH)Et.H2PO4. Laboratory supply catalogs routinely list it under its full IUPAC name for clarity, to avoid confusion with closely related tetrafluoroborate or chloride analogs. Seasoned researchers usually check for the correct anion, as swapping out phosphate for hexafluorophosphate or triflate can drastically alter both chemical and physical handling.
Safety & Operational Standards
Handling 1-hydroxyethyl-3-methylimidazolium dihydrophosphate reminds me of safer days in the bench-scale lab — no need for the same heightened vigil as with classic chlorinated solvents, but respect remains a constant. The literature reports minimal flammability, very low vapor pressure, and a notable reduction in acute inhalation hazards. Gloves, goggles, and properly ventilated areas make up the baseline for safe operation. Waste disposal generally follows local guidelines for phosphate-containing organic solutions, with less scrutiny than halogenated solvents. Small spills clean up easily with absorbent pads and water; the absence of strong odors or rapid evaporation lowers the likelihood of accidental exposure.
Application Area
This ionic liquid earns its stripes across various domains. Green chemistry advocates promote it as a solvent in biomass pretreatment and fractionation, letting researchers extract cellulose or lignin without permanent damage or hazardous byproducts. Electrochemistry labs use it for supporting electrolytes during organic electrosyntheses, achieving current efficiencies I rarely saw with traditional salt solutions. In materials science, this compound acts as a plasticizer and stabilizer for biopolymer films — students making edible packaging films have reported higher tensile strengths and greater clarity with this ionic liquid in the mix. In catalysis, it stabilizes transition metal complexes and speeds up esterification or transesterification, contributing to cleaner reaction profiles. Some pharmaceutical researchers experiment with this compound to solubilize poorly soluble active products or to streamline extraction of intermediates from fermentation broths.
Research & Development
Academic and industrial R&D have both invested serious energy into improving and understanding this class of ionic liquids. University laboratories look for novel applications, ranging from gas separation membranes to drug formulation vehicles. Startups and established firms scan for cost-effective preparation routes, trying renewable feedstocks and green chemistry principles. Multidisciplinary teams test the compound as a reaction medium for energy storage devices — batteries and supercapacitors that depend on ionic conductivity and negligible flammability. Analytical chemists also evaluate its use as a mobile or stationary phase in chromatographic techniques. Colleagues share stories about collaborative projects between synthetic chemists, material scientists, and engineers, all working to find the right balance of performance, safety, and affordability.
Toxicity Research
Researchers and regulatory bodies pay close attention to toxicity profiles, especially for new compounds touted as green alternatives. 1-hydroxyethyl-3-methylimidazolium dihydrophosphate fares better than many older ionic liquids. Cell viability studies often show minimal cytotoxicity at working concentrations. Biodegradation experiments point to partial breakdown in wastewater treatment, particularly when compared to persistent halogenated counterparts. Still, precautions persist — chronic exposure limits remain unestablished, so I’ve seen institutions recommend local exhaust ventilation and proper waste tracking. Ecotoxicity studies show slight aquatic toxicity, prompting recommendations for careful disposal and treatment of large-scale waste streams. Direct skin and eye contact can cause irritation, so the standard PPE kit stays on hand.
Future Prospects
Looking to the near future, the trajectory appears promising. Advances in sustainable chemistry rely on safer, more effective alternatives to harmful solvents, and this compound’s track record in biodegradability, low volatility, and versatile performance makes it a leading candidate for new industrial processes. As regulatory standards for solvent use tighten, demand for well-characterized, low-toxicity, easy-to-handle solutions continues to rise. Startups and multinational firms alike invest in scale-up technologies, while university groups push for fundamental insight into the interactions and long-term stability of ionic liquids in real-world conditions. Researchers still need expanded toxicity studies and more rigorous lifecycle impact assessments, but the trend toward safer, greener chemistry — paired with practical laboratory experience and innovative engineering — suggests this compound’s adoption will only expand in years ahead.
Delivering Real Solutions in Green Chemistry
1-Hydroxyethyl-3-methylimidazolium dihydrophosphate, a mouthful for most folks, catches attention not just for the name, but for what it actually does in the lab. Most scientists running reactions want safer, less toxic chemicals that get the job done and clean up easier than what their grandfathers used. This ionic liquid fits the bill as an alternative to some legacy solvents still floating around in fume hoods everywhere. Research teams, pressed by stricter safety rules and genuine worry over pollution, use it as a greener solvent in more synthetic projects each year.
The substance has drawn attention because it’s less flammable than older organic solvents. Nobody misses those fire risks that come with working late at night with acetone and other volatile liquids. The phosphate part means people wrestle less with disposal, which is a problem many labs face after big reactions. Companies don’t want fines or frantic phone calls from environmental inspectors. Chemists, in turn, appreciate a solvent that doesn’t stink up the building or damage rivers downstream.
Better Separations and Recovery in Industry
Industrial sites care about cost and efficiency as much as any university team thinks about safety. This ionic liquid’s knack for dissolving lots of materials, especially complex dyes or fine chemicals, gives it a regular spot in industry separation work. Textile factories don’t want dyes escaping into rivers. Plastic recycling plants want to break down complicated polymers without leaving behind junk. This chemical, designed for such tasks, pulls its weight, doing the hard part of pulling out specific components while leaving behind less residue that gets wasted or pollutes the pipes.
Energy matters too. Scooping out rare earth metals from electronic scrap looks simple on paper, but the real work happens when you need strong, specialized liquid solvents that pull out microscopic amounts from a stew of leftovers. Lab data over the past few years shows that ionic liquids like this one separate metals more efficiently than older liquid-liquid extraction processes, often with less heat or pressure. This means factories run cheaper and use less energy. Family-owned recycling operations see these savings directly; they can stay open—or hire more locals—instead of closing for failed inspections.
Practical Uses in Biotech and Pharmaceuticals
Pharmaceutical teams don’t gamble on new chemicals unless there’s a clear win. Some have turned to this ionic liquid to speed up the synthesis of drug precursors. It works well at low temperatures and doesn’t clog up reactors with sticky by-products. Less gunk means more reliable batch results and fewer shutdowns. Saving hours, sometimes days, on purifying a key compound becomes a big deal when medication supply links stretch thin.
Biotech entrepreneurs, including a few I spoke with at the last ACS meeting, start with this ionic liquid for enzyme reactions that need precise water content, which regular solvents mess up by drying things out or causing denaturation. Whether working on new enzymes for food processing or environmental cleanup, teams report less enzyme loss and higher product yield. It’s not perfect—costs remain higher than bulk solvents—but as patents run out and competitors look for improvements, some see this switch as just another smart upgrade.
Outlook and Real-World Problems
None of these uses suggest a one-size-fits-all solution. Lab managers worry about initial purchase price, while scale-up engineers run numbers on waste and recycling. Future research needs to tackle the recycling of these ionic liquids and the impact of any accidental spills before mass adoption sweeps through every factory. Policy makers, scientists, and industry heads should coordinate so safety, price, and environmental goals don’t compete, but work together. With better funding and regulatory alignment, adoption could speed up, and the benefits would show up not just in technical reports, but in the air and water around plants using these materials.
Understanding What Keeps a Product Safe
A lot of everyday products around us depend on chemistry—think medication, food, cleaning soaps, or even the sunscreen you use in summer. Chemical stability sets the clock for how long these things actually work as promised. The shelf life is not just a number on a label. It decides when a product starts to lose its punch, change color, or even become unsafe.
Nobody wants to take a drug and realize it no longer helps, or opens a tub of paint that’s turned to goo. Some chemicals break down when exposed to light, air, or moisture. Others react over time with what’s in the air. I remember learning in pharmacy school how aspirin, once opened, absorbs water and turns into vinegar-smelling powder. That smell means you’re not getting the pain relief you paid for. This isn’t only about comfort—stability influences health, money, and even trust in brands.
Main Factors Behind Stability
Formulation makes or breaks stability. The ingredients must play well together—one wrong mix and you could get clumps or strange smells that hint at chemical breakdown. Packaging comes next. Some vitamins break down fast in regular bottles, so companies use dark glass or foil pouches. For things like vaccines and insulin, even the tiniest temperature slip can wreck their structure.
People sometimes believe expiration dates mean products spoil the day after. That’s not the case for everything, but those dates are there for a reason. Food and medicine regulators order tough tests to see what survives heat, light, shaking, or months on a shelf. They open bottles every few months to check for ingredient strength. Even toothpaste has to prove it still cleans your teeth months later.
Real-Life Impact on Health and Business
Most don’t realize how stability reaches their lives. For example, hospitals have to throw out thousands of dollars’ worth of medicine because of short shelf lives. A batch of insulin, for example, degrades fast if left at room temperature, and patients who inject it may not even notice the loss in potency until they experience high blood sugar. Farmers buy pesticides by the barrel, but rainy seasons or heat waves can halve their effectiveness if the chemistry inside shifts.
Companies work with strict standards set by government agencies. The U.S. Food and Drug Administration, for example, expects proof that drugs or supplements keep their power and safety for a specific time. Companies tweak formulas and packaging, run stability trials for years, and collect real data about product changes.
Better Solutions for Longer Shelf Life
Some folks assume tossing things in a fridge keeps them fresh forever, but that trick doesn’t help everything. To lengthen shelf life, manufacturers look at new preservatives, airtight seals, or even smart packaging that changes color when contents go bad. There are also digital tracking systems so stores know when goods are about to expire. The more information we have, the fewer surprises at home.
Every product has a ticking clock built into it, starting the day it leaves the factory. Nobody wins if something spoils too soon—not the maker, the store, or the person who paid for it. By building chemistry knowledge into every step, companies protect both people and profits. Buying and storing products with an eye for stability pays off in safer homes and healthier lives.
The Chemical: Practical Caution Always Pays Off
1-Hydroxyethyl-3-methylimidazolium dihydrophosphate belongs to the ionic liquids family. It isn’t part of most household cabinets, but plenty of modern labs see it more often these days—used in catalysis, solvents, maybe some green chemistry research and process improvement.
These ionic liquids often get attention thanks to their low volatility and reduced flammability compared to volatile solvents. That doesn’t mean one should skip respect, skip the lab coat, or drop the goggles.
Storage: Cool, Dry, and Sealed Does the Job
Many might be tempted to think, “It’s not going to catch fire, so it can go anywhere.” That’s not the wisest route. Moisture turns into a real enemy for this compound. Even small leaks in a lab cabinet or a humid storeroom can lead to hydrolysis. The chemical could start to break down, shifting pH or releasing unwanted byproducts. That tinkering could wreck an experiment or taint a process, wasting time and research dollars.
From experience, simple precautions work best. An airtight container, preferably made from materials that don’t react with imidazolium salts or phosphate acids, stops the air and humidity from poking in. Keep the bottle tucked in a desiccator or a properly labeled cabinet, shielded from direct sunlight or sudden temperature swings.
Always keep the lid tight—not just for moisture, but also to keep out atmospheric CO2, which these salts sometimes interact with. Every cap left halfway open is a step closer to contaminated stock. Mark the dates on containers to track shelf life, since even well-stored chemicals have a best-before window.
Handling: Routine Safety, No Shortcuts
Gloves and goggles are non-negotiable, even if nobody’s outright allergic or sensitive to the material. I’ve seen small spills mushroom into bigger messes just from sticky fingers on unwashed lab equipment. Extra caution around acids and bases also matters, since mixing chemicals with the wrong residue on glassware brings up troubleshooting headaches nobody wants.
Scroll through material safety data sheets, but don’t just file them away. Refresh the team. Make sure everyone knows the immediate cleanup plans, how to spot contamination, where to reach the eyewash station, and where to store the chemical away from incompatible substances like strong oxidizers.
Most lab accidents don’t come from wild outlier events—they come from small lapses or distractions. The basics almost always save the day.
Fire and Accident Response: Prepare, Don’t Assume
No chemical storage system gets full marks without a fire plan. While imidazolium-based salts bring far less fire risk than most organic solvents, complacency still causes harm. Keep Class D extinguishers within reach because unexpected reactions do happen, especially in dynamic research labs where new mixtures get tested.
Dispose of even tiny samples properly. Clear containers, clear labeling, and routine disposal cycles prevent old chemical stockpiles from turning into unknowns down the line. Waste logbooks catch overlooked hazards before they sneak up on future lab staff.
Looking Forward: Smart Habits Build Trust
Colleagues trust each other when everyone buys into the same careful routine and keeps storage boring, tidy, accountable. The science and the people running those labs both depend on these details every single day.
Why Knowing What’s Hazardous Matters
Walk down any hardware store aisle and dozens of bottles flash bright warnings. A cleaner tucked under my grandmother’s kitchen sink had a skull and crossbones — it always caught my eye as a child. Years later, I started paying attention to those symbols after getting a whiff of ammonia mixing with bleach by accident. A lot of people only realize something’s hazardous after they’ve had a close call just like that.
There’s a reason folks in food and medicine put so much effort into clear labeling. Anyone can make mistakes, and a misjudged product at home or the workplace means someone could get hurt, or worse. Consumer Product Safety Commission reports show that thousands of accidents each year trace back to common chemicals, many of them misused or stored next to something incompatible.
Reading the Warning Signs
Hazard symbols and words like “caustic,” “flammable,” or “corrosive” do heavy lifting. These signs mean the product poses a specific risk. Flammable liquids catch fire from a stray spark. Something corrosive can burn through skin or ruin metal. Sometimes, the danger lurks in odors you can’t even smell: carbon monoxide, for instance, sends people to the ER with barely any warning. I once saw an HVAC worker ignore a “ventilate area” sign while refilling refrigerant; he passed out in minutes. Reading and respecting those warnings seems like such a simple thing, but it sets apart those who stay healthy from those who take a trip to urgent care.
Common Hazards at Home and Work
In my experience, people face more risk at home than they imagine. Bug sprays, pool chemicals, strong cleaners, solvents, and beauty products can cause problems if mixed by accident or left where a child can reach. At work, manufacturers must follow the Occupational Safety and Health Administration’s (OSHA) Hazard Communication Standard, which calls for labels and Safety Data Sheets (SDS) with everything hazardous. Workers depend on these sheets to guide storage, protection (gloves, goggles, and so on), and cleanup of spills. Issues pop up when these resources gather dust in a binder or get buried in electronic files.
Practical Steps for Safer Handling
Being careful doesn’t end at checking a label. Plenty of incidents happen with products that seem harmless because someone skipped the gloves or mixed chemicals in a hurry. PPE makes an enormous difference — it’s tough to remember, but a simple pair of nitrile gloves or a face mask blocks a surprising number of risks. Experts recommend storing products in their original containers, out of reach of kids and pets, and never combining products unless the label gives a green light.
People sometimes roll their eyes at these warnings, thinking they know better. I once spilled an industrial cleaner on my jeans, thinking ordinary denim was enough barrier — my thigh looked like it tangled with poison ivy for weeks. These mistakes sting and linger; they’re built-in reminders to treat these products with respect.
Real Solutions For Everyday Life
Fixing these issues starts with a healthy dose of respect for what we keep in our homes and workplaces. Asking questions at the store, checking company policies, actually reading the SDS before using something unfamiliar: that’s how we all reduce danger. No need for a chemistry degree, just a bit of curiosity and caution. Safer handling isn’t rocket science — it’s paying attention and following steps proven to prevent harm, and sometimes sharing stories like these to drive the message home.
The Real-World Impact of Chemical Purity
Chemistry runs through almost everything around us, from the medicine in a tablet to the plastics in household goods. The purity of a chemical compound shapes not only how it behaves in a process but also how safe the end product becomes. In my work, I’ve seen purity numbers shape decisions on a regular basis, and it’s rarely as black-and-white as a single percentage point. For a pharmaceutical ingredient, trace impurities can put a project on hold or force a product recall. In industrial manufacturing, unwanted residues impact yield, shelf life, and even worker safety.
Defining Purity Specifications
A typical purity specification goes way beyond one tidy number. Let’s take a common compound like acetaminophen. Pharmaceutical-grade batches must register at 99.0% or greater by most pharmacopeias, with every impurity—think p-aminophenol or 4-nitrophenol—kept under defined thresholds, often below 0.01%. Those numbers stem from toxicological studies and decades of practical knowledge about what contaminants could do. With research chemicals, you might see technical grade material at 95% or so, but for clinical or analytical use, 98–99.9% is the usual minimum. Sometimes, an extra 0.1% stands between reliable data and a failed research project.
Why Impurities Aren’t Just Numbers
Not all impurities weigh the same. Heavy metals, like lead or mercury, stand out as red flags even at trace amounts. Same goes for compounds like benzene, which remain unsafe far below the level of detection for basic methods. I remember a lab freezing distribution of a reagent after GC-MS turned up just a few parts-per-million of benzene—enough to trigger fresh safety checks, supplier audits, and a lot of paperwork.
Water content also matters. Many ionic or hygroscopic chemicals slowly pull in atmospheric moisture. This changes mass, affects reactivity, and creates confusion with downstream users. That’s why Karl Fischer titration or loss-on-drying data comes up together with the stated purity, especially in salts or hydrates.
The Role of Testing Methods
How labs verify purity shapes the results people trust. UV-Vis spectroscopy, HPLC, GC-MS—these tools reveal different things about a batch. A few times, I saw two suppliers both claim 99.5% by different methods. Only through running side-by-side testing did the real story emerge. Sometimes, a difference hides in what’s measured; one method might miss a low-level impurity that another picks up. Labs that provide detailed certificates of analysis with clear method statements offer real assurance.
Setting and Meeting Standards
The challenge shows up strongest in fields like pharma, food, or electronics, where an impurity can impact health, taste, or function. Regulatory bodies set strict standards. Companies who cut corners to save a few bucks on testing usually pay much more cleaning up problems later. On the other end, overly strict internal standards can raise costs without clear benefit. The best approach brings chemists, analysts, and buyers together to set those limits based on end-use, solid data, and some real world experience.
Solving the Purity Puzzle
To keep things moving forward, clear communication stands as the fix. Suppliers who give detailed impurity profiles—not just a single purity number—help downstream labs avoid surprises. Those details highlight whether a compound fits a medical, food, electronics, or industrial workflow, or if further purification is needed. Tech keeps advancing, making lower detection limits possible, but the key remains open data, well-trained analytical teams, and everyone along the supply chain talking honestly about what’s inside the bottle.