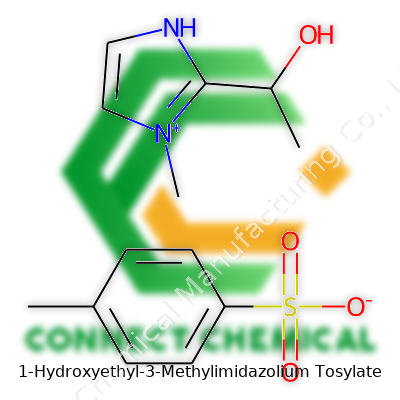
1-Hydroxyethyl-3-Methylimidazolium Tosylate: A Deep Dive
Historical Development
Curiosity drove chemists to explore ionic liquids over the past few decades, searching for solvents that could outperform water and organic options. In the early 2000s, 1-hydroxyethyl-3-methylimidazolium tosylate arrived at labs eager to exploit its unique features. Originally, the family of imidazolium salts saw more attention for their thermal stability, but as researchers looked for greener alternatives in synthesis and separation, these room-temperature ionic liquids caught eyes and never left the spotlight. The hunt for less volatile, non-flammable, and effective solvent systems put this compound in the conversation at conferences and academic journals. As new prep methods improved access and purity, industrial players also started taking notes.
Product Overview
This compound stands out thanks to a combination of imidazolium cation and tosylate anion. The cation usually comes with a hydroxyethyl group at the 1-position and a methyl at the 3-position. These two groups, paired with the aromatic sulfonate, give it a remarkable ability to dissolve both ionic and organic substances, making it a hit for applications where water falls short. Solid at room temperature or sometimes viscous, this ionic liquid skips the strong odor and volatility common in solvents from older textbooks. Its label often reads “1-(2-hydroxyethyl)-3-methylimidazolium tosylate,” but you’ll also see variations, covering a range of research and commercial sources.
Physical & Chemical Properties
Analytical work builds a consistent profile: appearance ranges from colorless to pale yellow, sometimes with faint brown tinges if impurities hitch a ride. It dissolves with ease in water, ethanol, and other polar solvents; its density usually sits between 1.2 to 1.3 g/cm³, though batch quality can nudge those numbers. Boiling isn’t its strength — it decomposes if pushed too far, so gentle heating serves best. Its melting range starts well below 100°C, favoring use at ambient or slightly elevated temperatures. Viscosity sits on the higher side, a sharp contrast to traditional organic ones. Chemical stability gets a boost from the aromatic sulfonate, limiting side-reactions, but strong bases or nucleophiles can attack, typically at the hydroxyethyl arm.
Technical Specifications & Labeling
Labels from suppliers track water content, usually below 0.5% for reliable results. Residual starting materials spell trouble, so NMR and GC check for these. Purity often exceeds 98%. Lot numbers, batch origins, and handling dates show on bottles, helping trace provenance. Storage guidelines caution against exposure to air or direct sunlight, since imidazolium rings don’t always appreciate long-term UV. Container size can jump from a few grams for lab-scale to kilogram units for industry, but secondary packaging stresses moisture prevention and leak resistance. Regulatory numbers, such as CAS, help match with compliance lists if necessary.
Preparation Method
Most labs follow a two-step synthesis: quaternization of 1-methylimidazole with 2-chloroethanol to produce 1-hydroxyethyl-3-methylimidazolium chloride, then metathesis with sodium tosylate to introduce the desired anion. Purification usually involves solvent washing and vacuum drying to drive off volatile leftovers. Scaling up remains straightforward — both starting materials come cheap and readily available, and the reactions tolerate a variety of solvents, from acetonitrile to water. Yields can exceed 80% with proper controls. Some researchers push cleaner green chemistry, avoiding chlorinated solvents and optimizing for waste reduction. Side-products rarely complicate this route, so analytical TLC or HPLC confirm the final compound with little fanfare.
Chemical Reactions & Modifications
Chemists love tweaking both the cation and anion for task-specific properties. Modifying the hydroxyethyl group, such as by etherification, shifts solubility and reactivity for cross-disciplinary projects. The imidazolium scaffold tolerates mild conditions, so appended functionality doesn’t demand exotic techniques. The tosylate can swap out with other sulfonates if reaction calls for it: the process repeats smoothly, and end-use drives any customization. As a solvent, it expands reaction windows for transition metal catalysis, biocatalysis, and organocatalysis. Grignard or strong base additions will eat away at the hydroxy functionality; acid-mediated reactions typically leave it alone, so process chemists pick their routes accordingly.
Synonyms & Product Names
Much of the literature refers to this ionic liquid as “HEMIm Tosylate,” a shorthand for easier page space. Product registrations list synonyms like “1-(2-hydroxyethyl)-3-methylimidazolium p-toluenesulfonate,” and in trade, it’s often just “Imidazolium Tosylate Hydroxyethyl Version.” Chemical supply catalogs sometimes shorten further, but the distinct pairing of hydroxyethyl and tosylate side-steps confusion with more common imidazolium bromides or fluorinated alternatives. Tracking the right variant matters, since swapping even a single anion or side chain shifts performance curve.
Safety & Operational Standards
Handling guidelines advise standard PPE: lab coat, gloves, goggles. Spills stay sticky and slippery, so steps for immediate cleanup focus on minimizing slips and contamination. The ionic liquid missed the list of major skin irritants, but mucous membrane contact should be avoided. Once, a colleague brushed a drop on exposed skin, reporting only mild redness after prompt wash-off. Fume hoods contain any aerosolized material during transfers; as a low-volatility liquid, inhalation risk takes a backseat compared to dusts or solvents with lower boiling points. Storage containers get sealed tightly, far from incompatible chemicals. Disposal follows local hazardous waste policies, typically entering organic liquid waste streams.
Application Area
Chemical processing demands versatility these days. This ionic liquid helps bring polar and non-polar worlds together, serving as a mediating solvent for tricky transformations in organic synthesis. Research groups have dissolved cellulose and other biomaterials that resist classical solvents, cutting steps in material modification. Catalysis, especially with transition metals or biocatalysts, benefits from its ability to stabilize reactive intermediates. Electrochemistry applications grew as its ionic conductivity matches or exceeds that of many traditional electrolytes, and its broad electrochemical window opens the door for new batteries and capacitors. Its role in selective extraction for environmental analysis makes it part of the green chemistry conversation. Scale-up challenges linger, but the speed of academic and industrial adoption reflects more than trend-chasing.
Research & Development
Journal databases keep growing with reports testing 1-hydroxyethyl-3-methylimidazolium tosylate in new roles: catalyst supports, biomass processing, analytical separations. Funding bodies focus on ionic liquids for their potential to cut emissions and waste. Recent papers tracked solubility improvements for pharmaceuticals and agrochemicals, melting previously stubborn compounds in one-pot setups. Customization, either by new cation building blocks or by trading out the tosylate, broadens its research impact. Many still experiment with process intensification—shortening synthesis times, recycling solvents, and integrating with continuous-flow reactors—to edge closer to large-scale commercial viability.
Toxicity Research
Toxicology studies for this class of ionic liquids stress both acute and chronic exposure. Results on 1-hydroxyethyl-3-methylimidazolium tosylate mostly fall in the moderate-to-low toxicity category, but effects depend heavily on dose and exposure route. Cell line studies show some cytotoxicity at higher millimolar concentrations; aquatic assays flag potential risks for certain organisms if discharge isn’t controlled. Standard regulatory testing continues, and environmental breakdown rates favor longer persistence than ethanol or acetone, putting pressure on end-users to manage waste and prevent runoff. Calls for more in vivo data get louder, especially as new industries eye large-scale usage with human or ecological exposure potential.
Future Prospects
Ionic liquids won’t replace every solvent in sight, but their unique blend of properties keeps pushing boundaries. As regulatory frameworks tighten around traditional organics and more sectors chase renewable, safer options, compounds like 1-hydroxyethyl-3-methylimidazolium tosylate look appealing for the long haul. Improvements in manufacturing—cheap, low-waste routes and better recycling—could drive down costs further. Researchers aim for better toxicity profiles or full biodegradability without giving up stability. Real progress will keep coming from collaborations between industry and academia, where honest data about risks and performance cuts through the hype. Every new use case uncovers both promise and practical hurdles, but real-world feedback has always been the fastest catalyst for improvement.
A Closer Look at an Important Ionic Liquid
1-Hydroxyethyl-3-methylimidazolium tosylate often pops up in labs where green chemistry and sustainable processes matter. The rise of this ionic liquid isn’t just a fad—scientists and innovators keep turning back to it for several good reasons. Its main draw comes from the way it cleanly dissolves a wide array of organic and inorganic compounds. In my own experience, shifting to greener solvents made a real difference to the environmental footprint of routine chemical research, and this substance played a part in that.
Driving Greener Chemistry
Most chemists recognize the struggle: fossil-derived solvents litter the industry and create hazardous waste. 1-Hydroxyethyl-3-methylimidazolium tosylate shows up as a non-volatile, reusable option. Its negligible vapor pressure means fewer toxic fumes, slashing the need for energy-guzzling fume hoods and lengthy safety reviews. This trait makes it a favorite for both benchwork and scaling up. Several peer-reviewed studies confirm its ability to support cellulose and lignin extraction processes—something old petroleum-based solvents struggled with.
Enabling Advanced Synthesis and Catalysis
With this ionic liquid, scientists gain more control over reaction rates and solubility in everything from organic syntheses to specialized catalysis. A colleague once used it for selective alkylation and shared that side products dropped drastically, whittling down post-reaction cleanup. The ability to recycle the solvent without major loss of performance saves both time and resources. Manufacturers keep looking for ways to reduce chemical waste, and this liquid fits in by staying stable under a range of conditions and being easy to separate once the reaction finishes.
Shaping Biomedical Research
Researchers in drug design and delivery also value 1-Hydroxyethyl-3-methylimidazolium tosylate for its predictable interactions with biomolecules. Because it can dissolve both polar and non-polar compounds, it gives a clear route to making and purifying complex pharmaceutical intermediates. Data from academic labs show that this ionic liquid supports high-yield synthesis of peptides and nucleosides. On a practical note, switching to non-traditional solvents often means a learning curve, but user reports suggest this substance handles much like water but with more chemical flexibility.
Challenges and Responsible Use
As promising as it sounds, nothing is perfect. While toxicity levels fall below many traditional solvents, every lab and plant using 1-Hydroxyethyl-3-methylimidazolium tosylate needs to run full safety checks—good practice for any industrial chemical. Disposal routes require careful planning, and regulatory bodies continue to study long-term impacts. Large-scale adoption hinges on open data about biodegradability and human health outcomes. Calls grow louder for companies to take transparent approaches and share findings, rather than bury results in company reports.
Looking Forward
Current evidence and day-to-day observations point toward ionic liquids like 1-Hydroxyethyl-3-methylimidazolium tosylate standing at the center of safer chemistry. Ongoing research fuels optimism that one day labs can offer not only strong results but also cleaner, safer environments. Scaling down reliance on hazardous materials calls for teamwork between labs, chemical suppliers, and regulators. With responsible oversight and honest communication, the hope is to move toward real sustainability—one successful experiment at a time.
Digging Into the Chemical Make-Up
1-Hydroxyethyl-3-Methylimidazolium Tosylate, often dubbed [HEMIM][OTs], brings some real character to the world of ionic liquids. Its chemical formula goes as C8H15N2O3S for the full salt, combining the cation and anion into one neat package. The two main pieces, the cation and the tosylate anion, give this compound both personality and surprising stability.
Start with the cation, 1-hydroxyethyl-3-methylimidazolium. The core rings with imidazole: a five-membered structure featuring two nitrogens, giving the backbone some pretty unique electronic properties. One nitrogen wears a methyl group—simple but effective, adding a touch of nonpolarity. On the other nitrogen, a hydroxyethyl group brings both polarity and hydrogen-bonding ability. That combo opens doors for solubility in water while letting the compound play well with other solvents like DMSO or ethanol.
Pair that cation with a tosylate anion (p-toluenesulfonate, C7H7SO3–), and you get a salt which dissolves quickly, even in settings where more traditional salts won't budge. The aromatic ring from the tosyl group throws in some extra character. That structure lets the anion fit nicely into the cation’s environment, stabilizing it and giving the whole molecule a staying power that chemists and engineers prize in both research and industry.
Why This Structure Matters in Everyday Science
Anyone who’s spent time in a chemical lab has seen solvents that just won’t behave—either too reactive, or leaving residues that take forever to clean out. 1-Hydroxyethyl-3-Methylimidazolium Tosylate flips this on its head. The combination of the hydroxyethyl group and tosylate creates an ionic liquid with low vapor pressure, meaning it doesn’t just disappear into thin air or create safety headaches. It tolerates air, water, and the heat of vigorous reactions.
This salt’s ionic nature gives it a unique leg up in catalysis, electrochemistry, and separations, especially in sustainable chemistry. With the industry-wide shift to greener solutions, more researchers look to these less volatile, customizable solvents. No surprise—they’re easy to recycle and don’t bring along the same environmental baggage as traditional organic solvents.
Real-World Applications: Not Just Lab Curiosity
Having spent late nights running organic reactions, I’ve learned firsthand the pain of lost yield because a reaction mixture dried out or picked up water. Ionic liquids like this one can help there. They keep reactions going at steady rates, and that reliability can trim costs in pharmaceuticals or advanced materials manufacturing.
One challenge: price and accessibility. These specialty salts often cost more and need careful quality control. But with growing demand comes better supply chains and more affordable production. Researchers who team up with chemical suppliers sometimes negotiate bulk deals or work together on greener manufacturing methods, cutting down both price and waste.
Paths to Safer, Smarter Chemistry
For chemists aiming to do better—on safety, on waste, on performance—compounds like 1-Hydroxyethyl-3-Methylimidazolium Tosylate matter. They can replace volatile, toxic solvents and improve efficiency, freeing up time and resources for the work that actually moves science forward. Collaboration between academia, industry, and suppliers keeps driving improvements, making advanced molecules like this one more available and affordable.
Every step toward smarter solvent use keeps labs safer and production greener. When more researchers learn about and trust these compounds, the industry as a whole shifts toward practices that respect both science and the planet. That kind of change makes a bigger difference than any one molecule could on its own.
Why Details Matter in Chemical Storage
Anyone who deals with chemicals knows experience counts for a lot. Plenty of good labs have lost an expensive sample—or worse, had a close call—just because someone underestimated hygroscopic liquids. 1-Hydroxyethyl-3-Methylimidazolium Tosylate stands out among ionic liquids for this exact reason. This compound will pull water right out of the air if left open, and that changes both the purity and how well it works in synthesis or electrochemistry.
I remember opening a fresh bottle too slowly—moisture in the air made the top sticky in a single afternoon. That sticky residue usually signals contamination, and contamination can wreck a controlled experiment. Research journals back this up: a single percent of water in this material, and the physical properties begin to drift out of spec. Yield drops and reproducibility fades away.
Physical Hazards and Worker Safety
Most people forget how little it takes to breathe in a trace amount or splash some on their skin. This compound can irritate skin and eyes; nobody wants to start a workday at the eyewash station. From my own time at the bench, I can tell the importance of basic safety gear—nitrile gloves, splash goggles, and a cotton lab coat every time. Read the SDS, but don’t just file it away in a binder.
Good ventilation also keeps the nerves calm. 1-Hydroxyethyl-3-Methylimidazolium Tosylate does not have a sharp smell, so a fume hood should always stay open while handling it. Spills find every crack, so keep absorbent pads on standby and label everything clearly to prevent confusion during a busy day or shift change.
Storage Conditions
Find a spot away from direct sunlight and any heat source. I always prefer amber glass over plastic for these ionic liquids. The plastic may leach or allow slow moisture intrusion over weeks. If a refrigerator or cool, dry cabinet stands available, use it. Don’t freeze the compound without checking if crystallization will create handling issues. Good temperature control preserves shelf life and prevents viscosity changes that make accurate pipetting a real chore.
Even a well-sealed container can fog up if it comes from a cold fridge to a humid lab. I always return the bottle promptly after pouring, then wipe down the exterior just in case. Parafilm helps, but it never substitutes for a proper sealing cap.
Inventory and Management
Track batch numbers and acquisition dates with a permanent pen. A lot of labs lose money buying new stock because an old bottle sits forgotten at the back, useless because it’s clumped and discolored. I mark down every withdrawal and make sure to rotate stock. Regular checks, simple checklists, and an organized chemical inventory keep both safety and budgets in line.
For disposal, ask the environmental team before pouring anything down the drain. Ionic liquids hold up well against water treatment, meaning small spills can linger in wastewater unless caught at the source. Containerize leftovers and label for hazardous waste pickup.
Improving Daily Habits
Success with 1-Hydroxyethyl-3-Methylimidazolium Tosylate comes from attention to daily habits: quick, clean handling, limiting air exposure, and respecting the hazards with the right safety equipment. Training new researchers to stay sharp makes everyone’s work easier. A sticky cap or a ruined batch does more than just cost time; it teaches that details make all the difference in chemistry.
Getting to Know This Chemical
1-Hydroxyethyl-3-methylimidazolium tosylate isn’t something found on household shelves, but it appears in labs and some specialty industries. This ionic liquid often pops up in research on green chemistry, because people see it as less hazardous than regular solvents. That gives it a reputation for being friendlier to both users and the environment. Still, researchers and workers question if it really deserves that reputation.
Examining the Safety Claims
People like to say that ionic liquids are safe, but that doesn’t mean every chemical in the class gets a free pass. Looking at its safety data, there are no wild warnings about flammability or explosions, which is a relief for lab workers. Yet, reports do note eye and skin irritation. If you’ve ever caught a whiff of strong cleaning products and felt your nose tingle, that’s the kind of reaction you might get without proper gloves and glasses.
Researchers in Europe pointed out that the tosylate part might cause allergic reactions if you have sensitive skin. There is also discussion about respiratory irritation from inhaling dust or vapor. Some animal studies flagged mild toxicity at higher doses, but it didn’t rank among the worst offenders. On the bright side, it breaks down faster than older solvents when left to the elements—nature takes care of it better than it handles some old-school chemicals.
What Science Says About Exposure and Environment
I see why green chemistry circles cheer for this compound. It hardly evaporates, which means breathing in significant amounts during routine lab work stays unlikely. That’s a relief compared to the headaches and nausea I’ve seen after spills with volatile organic solvents. Environmental toxicity gets attention, too. Fish and algae can react badly if the chemical lands in water, but at much higher concentrations than common lab spills produce.
Some papers run toxicity tests on worms and other soil dwellers. The results aren’t perfect, but they don’t suggest mass die-offs. The real risk comes from large releases, not the small volumes people use in research or manufacturing. I think about the times chemical waste rules got ignored—and realize that following disposal guidelines is where the real protection sits. Most companies have these rules in black and white, so there’s little excuse for dumping leftovers in a sink.
Keeping Things Safe: Good Habits and Policies
If this chemical appears in your workspace, the right response isn’t panic—it’s respect. Grab gloves, pop on glasses, and work in a fume hood just like the safety sheet says. I once broke a bottle of ionic liquid and learned that cleanup is slow because the stuff is thick and sticky. Nobody suffered long-term harm because my lab stuck with basic practices and called the safety officer for help.
I push for companies to give decent safety training, not just a handout nobody reads. I trust the science, but I trust habits more. Regulators in Europe and the US check new chemicals and sound alarms if trouble appears. They already ask for visible labels and proper containers, so nobody mistakes this for harmless soap. Research continues, so if toxicity tests ever turn up new risks, safety rules can adjust fast.
Balance and Precaution
No chemical scores a perfect safety grade. 1-Hydroxyethyl-3-methylimidazolium tosylate looks less scary than many older substances, though. With the right habits—gloves, goggles, and smart disposal—I wouldn’t lose sleep over it. My experience tells me that most problems come from ignoring good practice, not mysterious dangers hiding in the bottle.
Breaking Down the Real Value
Laboratories and chemical plants keep searching for ways to push reactions further or speed them up. Scientists want solvents that go gentle on the environment but stay strong in performance. For those of us who work with ionic liquids, 1-Hydroxyethyl-3-Methylimidazolium Tosylate (HEMITsO) has shown steady potential. This salt-like liquid grabs attention because it sheds many of the problems tied to older chemical solvents.
Stepping Past Old Solvents in Synthesis
Regular organic solvents come with flammability concerns and stubborn waste. HEMITsO, on the other hand, almost refuses to evaporate, so labs lose less to the air. While working in an academic setting, I saw researchers reach for this ionic liquid during challenging organic reactions, like the synthesis of esters or other functional group transformations. They found that it let reactions roll on at moderate temperatures, dragging out higher yields and fewer by-products. Safety officers also liked that spills didn’t fill the air with fumes.
Catalysis: A Better Platform
Catalysts need support, something that doesn’t get in the way. This is where HEMITsO shines. Its chemical structure offers a stable ground for both enzyme and metal-based catalysis. Colleagues in green chemistry often brought up success stories in cross-coupling and alkylation. They saw improvements in both speed and selectivity with this ionic liquid, especially when recycling catalysts or running them in continuous flow setups.
Electrochemistry’s Dependable Friend
Electrochemistry counts on solvents that let ions hustle. Not every liquid fits the bill. HEMITsO carries ions well, standing up under voltage and temperature swings, which makes it attractive for things like plating metals, developing supercapacitors, or even novel battery prototypes. People at energy startups look for liquids that don’t age fast or corrode equipment, and this one checks the right boxes.
A Greener Option for Extraction
Extracting compounds out of plant, food, or soil samples typically needs copious, nasty solvents. HEMITsO offers a workaround. Its structure pulls either polar or non-polar substances, so it serves scientists blending up botanicals or analyzing soil for pollutants. In my experience, extraction setups ran cleaner, yielded more, and cut down on solvent-related headaches, since the ionic liquid could sometimes be recovered almost fully for another round.
Solvent for Polymers and Advanced Materials
HEMITsO works for dissolving cellulose and other stubborn biopolymers that won’t break in regular organics. This helps researchers build films, membranes, or hydrogels for medical and industrial purposes. Modern textile labs pay close attention because redesigning processes with less waste and smaller ecological footprints grows more important each year.
Looking at Solutions
People see these advances and start asking tougher questions about price, long-term health impacts, and the practical side of using ionic liquids outside the lab. It matters that teams openly report what they run into — waste management issues, compatibility with equipment, and the rare but notable cases of toxicity. If everyone shares both the gains and the dead ends, industry and research benefit. The future likely involves blending traditional solvent wisdom with these new options, guided by research that favors transparency and real-world evidence.