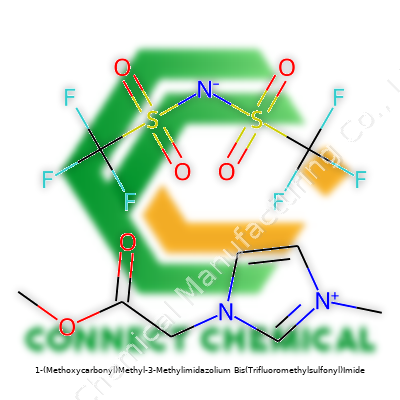
1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide: In-depth Commentary
Historical Development
Liquid salts caught the eye of researchers in the late twentieth century. No one could ignore their potential for tuning solvent properties while cutting out volatile organic compounds. A group of chemists put their focus on imidazolium salts, thanks to unique stability and versatility. The path from standard methylimidazolium salts to more specialized versions, such as 1-(Methoxycarbonyl)methyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide, reflects an evolution driven by efforts to reduce environmental impact, boost ionic conductivity, and expand Compatibility in applications like electrochemical devices and catalysis. This was not a story of one lab’s victory—every gain built on hundreds of rounds of synthesis, toxicity testing, and real-world setbacks.
Product Overview
This ionic liquid stands out for its dual functional tails: a methoxycarbonylmethyl group attached at the N1 position and a methyl group at N3. The bis(trifluoromethylsulfonyl)imide anion lends the stability that heavy industry and academic groups both lean on. In practice, this means a salt that melts around room temperature, tolerates moisture, and stays liquid over a larger temperature range than typical molecular solvents. Electrochemists prize it for making energy storage systems safer and more robust. Synthetic chemists reach for it because it can dissolve many organic and inorganic compounds, giving new options for difficult transformations.
Physical & Chemical Properties
1-(Methoxycarbonyl)methyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide usually appears as a clear to slightly yellowish viscous liquid. Density often hovers near 1.4 g/cm³. It brings low volatility—almost no smell hits the nose—and stays liquid even when it cools well below zero Celsius. Its ionic conductivity runs between 5 and 10 millisiemens per centimeter, depending on preparation specifics and how much water gets in. Thermal stability stretches up past 350°C, so researchers interested in high-temperature electrochemical work keep returning to it. That trio of carbon, hydrogen, and fluorine-rich units shapes both the electrical profile and hydrophobic balance. The anion, with its large size and delocalized charge, helps separate ions and keeps viscosity relatively low for an ionic liquid.
Technical Specifications & Labeling
Bottles arrive with precise labeling. Typical content exceeds 98% purity, proven by NMR spectroscopy and elemental analysis. Moisture percentages rarely pass 0.1%; most suppliers use vacuum-drying to reach this level. The product passes IR and mass spectrometry checks for residual synthesis byproducts. Many researchers—myself included—prefer lot-specific data sheets confirming moisture and halide content on each sample. Packaging uses glass or HDPE bottles, sealed to keep water out. Labels note the CAS number, molecular formula C11H13F6N3O5S2, and hazard symbols for skin and eye irritation. Because the cation carries an ester tail, labeling includes shelf-life recommendations to help avoid unintended hydrolysis or decomposition.
Preparation Method
Small labs and bulk producers tend toward a two or three-step approach. Starting from 1-methylimidazole, the methoxycarbonylmethyl group attaches via alkylation using methyl chloroacetate and a base, typically potassium carbonate, in a polar aprotic solvent like acetonitrile. The intermediate chloride salt goes through anion exchange by treatment with lithium bis(trifluoromethylsulfonyl)imide in water, driving out the highly soluble lithium chloride. After phase separation, repeated water washes remove leftover lithium and halides, and the final product dries under vacuum at elevated temperatures. Labs careful about byproduct removal sometimes add charcoal filtration or perform column chromatography. This route shows how adaptation in process steps matters when switching from milligram-scale curiosity to kilogram-scale supply.
Chemical Reactions & Modifications
The methoxycarbonylmethyl group makes this salt ripe for post-synthetic modification—not something all ionic liquids offer. Given gentle heat and base, the ester can hydrolyze to yield a carboxylate, giving users a route to switch the hydrophilicity or tweak binding affinity for metals. The methyl group at the third position stays inert, resisting side reactions during most organic or organometallic procedures. The bis(trifluoromethylsulfonyl)imide anion rarely interferes, showing strong stability even with aggressive Lewis acids or electrochemical events. Over the years, I’ve seen colleagues add alkyl, benzyl, or even chiral auxiliaries to this imidazolium core, aiming to create task-specific ionic liquids for asymmetric catalysis. The backbone holds up.
Synonyms & Product Names
More than a few ways refer to this compound: CMMImNTf2, 1-(methoxycarbonyl)methyl-3-methylimidazolium NTf2, or methylimidazolium methylcarboxylate bis(trifluoromethylsulfonyl)imide. Chemical supply catalogs differ in listing abbreviations, but the imidazolium-NTf2 tag always points in the right direction. Brand names sometimes appear in research literature, and users working across labs need to double-check structures to avoid confusion with similar-sounding products or related cations.
Safety & Operational Standards
Ionic liquids often carry a reputation for safety, but this doesn’t mean anyone should get careless. Direct skin or eye contact leads to irritation—much like many other imidazolium salts. The trifluoromethanesulfonyl fragments call for particular attention; inhalation of fine aerosols irritates respiratory passages and can produce unpleasant reactions. Nitrile gloves, splash goggles, and lab coats offer enough protection for weighing and mixing. Fume hoods matter for large-scale operations, especially if warming the liquid or handling powders. Any spill sticks, so lab groups keep ethanol and absorbent wipes nearby. Disposal routes focus on incineration—avoid drains because ionic persistence in the environment frustrates wastewater treatment and aquatic organisms. Manufacturers hold themselves to REACH and OSHA guidance, providing SDS sheets for any workplace using more than a few grams.
Application Area
Battery labs snapped up this salt as soon as they realized its robust electrochemical window—up to 5V—let them move beyond lithium hexafluorophosphate systems. The liquid’s hydrophobicity and chemical stability support work in supercapacitors, organic electrosynthesis, and electrodeposition of metals that fight with water or traditional media. In my own work with homogeneous catalysis, it provides a genuinely inert solvent. Transition metals, even those notoriously sensitive, last longer, and product isolation gets easier, because simple extraction with nonpolar solvents removes products without dragging out the ionic liquid. Polymer chemists work it into ionogels and develop membranes for gas separation. Enzyme immobilization and controlled-release matrices also keep popping up in academic papers—suggesting biotech and pharmaceutical factories could widen adoption.
Research & Development
Open a recent chemistry journal, you’ll spot this salt in protocols searching for green alternatives to old solvents. Battery teams keep testing it for longevity, cycle stability, and dendrite resistance, especially as the world looks beyond lithium to sodium or magnesium ion technologies. Organometallic chemists chase more efficient coupling reactions or metal recovery from electronic waste. Research never stops at a linear path. Colleagues in analytical chemistry love its suppressive influence on matrix effects, producing smoother mass spectra and improving detection in complex mixtures. The feedback cycle between bench, pilot plant, and tech scale drives new derivatives every year—with each tweak promising a better solution for either environmental regulations, cost, or performance.
Toxicity Research
Early reviews paint a nuanced picture. Acute toxicity ranks low in most models—rodents survive fairly high oral or skin exposures, and in vitro cytotoxicity lands well below many traditional solvents. Chronic studies warn about endocrine disruption and aquatic impact, as these salts linger. The NTf2 anion, resistant to breakdown, may accumulate if released in bulk. Most incidents—spill exposures, accidental splashes—produce irritation instead of systemic illness, based both on literature and anecdotes from labs. The challenge comes from predicting fate in waste streams or air. Ongoing research looks at biodegradation, microbial processing, and lifecycle analysis. Safety teams argue that the learnings from ionic liquids can’t stop at acute screening—a claim I’ve come to respect, as safety culture evolves quickly when real incidents occur.
Future Prospects
The appetite for stable, tunable ionic liquids won’t disappear. As green chemistry standards rise, early adopters of this salt keep publishing new applications involving energy storage, CO2 capture, and industrial catalysis. Continued efforts in synthesis may create versions with reduced fluorine content or tailored for easier downstream processing. As regulations on hazardous solvents tighten, industry looks for drop-in alternatives. My experience says that good communication between synthetic chemists, engineers, and environmental scientists will shape which ionic liquids step into the spotlight. Seeing global investment in sustainable chemistry, this compound holds enough flexibility to adapt to new challenges—for chemical manufacturing, for electronics, for materials science, and much more.
A New Type of Tool for Chemists
Most folks haven’t heard of 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide, but anyone working in advanced chemistry or clean technology likely knows its value. Chemists call it an “ionic liquid,” meaning it’s a salt that’s actually a liquid at room temperature. This makes it pretty useful where scientists need a solvent that's stable and non-volatile. People like me, who’ve spent too many late hours in a lab, know that solvent choice shapes the results before any reaction even kicks off. Ionic liquids have this knack for swapping out traditional, more harmful solvents, helping researchers keep the air and workspace free from harsh vapors.
Changing the Way Chemical Reactions Happen
This compound steps up in areas where water or regular organic solvents just won’t cut it. I remember running a stubborn synthesis, one that just kept failing with standard methods. Someone suggested using an ionic liquid, and it surprised me how smoothly the reaction went. This compound in particular holds on to its liquid state across a wide range of temperatures. It doesn’t evaporate or break down easily, so people use it in demanding situations: electrochemistry, battery research, and even catalysis. It gives these reactions a better environment, boosting yields and making the process cleaner.
Supporting Greener Alternatives for Industry
Sustainability means more than just talk—labs and manufacturers need real, practical options. The old petroleum-based solvents often come with environmental baggage, from hazardous waste to air pollution. Substituting those for this type of ionic liquid cuts down on emissions and toxic byproducts. Regulations on pollution keep getting tougher, so companies lean on these modern solvents for cleaning up their act. Years ago, you’d see barrels of hazardous waste outside a plant. As green solvents catch on, waste drops and costs actually go down, especially when solvents can be recycled.
Key Player in Next-Generation Batteries and Electronics
People working on batteries—think lithium-ion or new solid-state types—have caught onto the value here. Ionic liquids like this one carry ions between electrodes but don’t catch fire easily, so they go into safer battery prototypes and other electronic components. Unlike water, they don’t short out the system. Unlike some old-school choices, they won’t blow up if a little moisture sneaks in. I’ve seen research teams extend battery life and avoid overheating just by picking the right electrolyte. When technology demands reliability and safety, chemistry provides the leverage.
Facing Costs and Recycling Challenges
No chemical comes without tradeoffs. Ionic liquids take energy and resources to make, and not every plant already has the recycling systems that make reusing solvents practical. When I started in the lab, extracting products from ionic liquids felt slow and tedious. Over time, researchers and engineers developed better systems to separate out the reused material. Costs come down the more you use them, but widespread adoption only happens as companies see clear savings and lower risk.
Moving the Field Forward
Talking with colleagues, there’s a clear hunger for solvents that solve more than one problem at once: safety, efficiency, and environmental footprint. 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide stands out for its tough, reliable profile. Every new study or product built around these ionic liquids makes it easier to leave behind toxic, old-fashioned solvents. The more researchers learn, the closer we get to greener labs and safer products on shelves.
Digging Into the Details
Walking into a laboratory for the first time, I felt a little lost staring at bottles flashed with obscure codes: C2H5OH, H2SO4, CaCO3. These are not just fancy strings for chemists to remember—they provide a kind of fingerprint for every compound. Understanding what a chemical formula tells us can open up how and where we use a substance. C2H5OH? That’s common ethanol. Every chemistry teacher will stress its formula precisely because any small change gives you a different substance with a different set of risks and benefits. CH3OH, for example, is methanol and a dangerous toxin.
A chemical formula tells you the atoms in a molecule and the exact proportions. Look at sodium chloride as NaCl. This just means for every sodium atom, you get a chlorine atom. Simple as cooking with a recipe—mess up the ingredients, and you don’t get what you expect. Now, if you need to make a saline solution, you choose NaCl and never NaBr. Early on in my classes, I once confused salt with sugar (NaCl and C12H22O11), and what should have been an easy experiment turned into a sticky mess. Knowing the formula guards against embarrassing mistakes—and sometimes, life-threatening ones.
The Power of Molecular Weight
Molecular weight, or molar mass, is another side of the coin. Every time I stood at a balance, measuring out how much of a compound to dissolve, I needed its molecular weight. Missing this step might waste expensive chemicals or throw off the results. For sodium chloride, the molecular weight runs about 58.44 g/mol. That number comes from adding up the atomic weights listed on the periodic table for sodium and chlorine. Balancing precise doses in medications, fertilizers, or industrial cleaners all starts here.
Errors in calculations can spiral quickly beyond wasted resources—think about a nurse who mixes up doses or an engineer overestimating what a chemical can handle in a pipe. Even the wrong label could turn a school science lab from a place of learning to a hazard zone. Precision saves lives and money. Having formulas and molecular weights in hand means better decisions from the beginning.
Building Trust Through Transparency
Knowing what's in a bottle matters well outside labs. As a consumer, it always mattered to me that ingredient labels listed real formulas. Is that vitamin supplement truly vitamin C or something slightly off? Public trust grows with clear, accurate chemical information. It's the backbone of how companies prove safety and comply with regulations. I recall a story where a manufacturer mislabeled cleaning agents, and people ended up with severe skin reactions. A transparent chemical identity would have protected everyone involved.
Solving the Issue of Mislabeled or Unknown Compounds
One solution stands out: better education. Many disasters in chemistry boil down to confusion about formulas. Teaching people not only what a formula looks like, but how to use it in real-world situations, builds competence and confidence. Companies and schools owe it to staff and students to train them in reading labels, calculating dosages, and using molecular weights with confidence.
Another piece involves adopting digital tools. Barcode scanners linked to chemical databases cut down labeling errors and speed up ingredient verification. I’ve seen labs save hours and avoid accidents this way. In hospitals, pharmacists check molecular weights before compounding drugs to ensure no mix-up sneaks through. Moving information from paper to accessible, updated databases helps everyone involved make better choices.
Paying Attention to Safety Means Protecting More Than Your Product
Many of us have gotten used to the idea that safety rules are just for compliance. Maybe a sign goes up near the shelf or a dusty binder waits on a back office desk. Experience tells a different story. Safe storage and handling shape the wellbeing of workers, families, and communities. Let something go wrong, and the fallout lands on real people, not just on spreadsheets.
Getting Storage Conditions Right
Temperature stands out as a foundation for safe storage. Every product comes with preferred temperature ranges, often noted on packaging or in technical documents. Chemicals might break down or become hazardous under heat, while medicines can lose power if the air gets too warm or cold. I’ve walked into storerooms and found sensitive material sitting next to a sunny window—nobody thought much of it, but all it takes is one heatwave for costly damage. Thicker insulation and well-placed thermometers can cut those risks in half.
Humidity matters, not just for powders and biological items. Even hardware like metal tools and electronics fail if they pull in water molecules from damp air, setting off corrosion that you don’t see until something stops working. Desiccant packs and sealed bins don’t cost much, but they pay off quickly. I’ve seen an entire shipment of inventory written off because someone skipped this step.
Separation and Labelling—Not Just for Show
It’s tempting to stack everything in the closest available corner, but mixing up incompatible goods puts everyone at risk. Chemicals, in particular, should never share shelving with food, drink, or materials they might react with. It only takes a drip or a tipped bottle to spark a dangerous reaction. Clear, sturdy labels reduce confusion, especially when multiple people take turns handling supplies. During a storm, the fastest responders are often the ones who can see facts at a glance, not after a puzzle.
Access and Training: Everyday Practices That Save Lives
Locked cabinets and clear access rules keep curious children, pets, or visitors away from danger. Where I’ve worked, everyone gets a rundown on what goes where on their first day—no exceptions. Refresher sessions catch up with habits and encourage everyone to report mistakes instead of hiding them. Regular training builds muscle memory and a sense of ownership that formal policies alone can’t guarantee.
Dealing With Emergencies: Planning Ahead
No one plans to have a spill, fire, or leak, but drills and clear escape routes keep a bad day from turning tragic. After a minor chemical issue, I saw how quick thinking and ready supplies kept a problem contained. The right gloves, eyewash stations, and cleanup tools do more than tick boxes—they keep accidents small and manageable. Posting clear instructions in plain language next to storage areas prevents panic and builds confidence.
The Role of Documentation
A simple checklist or logbook can make a big difference. Tracking who uses and moves items limits confusion, helps find missing supplies, and even catches problems before they spread. Digital systems make it easier to update and share records, which is handy when dealing with vendors or local authorities. Logs helped me spot patterns that led to smarter purchasing and fewer wasted materials.
Looking Forward
Safe storage and handling rarely draw attention, yet they form the backbone of a healthy work or home environment. Skipping steps may save time at first, but always leaves room for trouble. A careful setup, some thoughtful tools, and a commitment from everyone create protection and peace of mind—no cutting corners needed.
What Makes an Ionic Liquid Blend In—or Stand Out?
Step into any modern chemistry lab and you’ll hear talk about ionic liquids. These materials pop up everywhere, from green chemistry projects to advanced batteries. They promise a lot, so it’s helpful to look closer at how their solubility and compatibility shape where, and how, they actually get used.
Solubility: More Than Just “Dissolves in Water”
Solubility isn’t just about whether something mixes with water. Ionic liquids stand out because their solubility shifts with tiny changes to their structure. Swap out a cation or anion and suddenly, you’ve got a liquid that dissolves polar organics or, just as easily, stubborn oils. That chameleon-like nature makes them handy in all sorts of chemical jobs. In my work, mixing these liquids with cellulose or natural extracts, I’ve seen them create solutions where regular solvents barely make a dent.
Beyond the lab, this matters. Big companies look for safe ways to separate metals or recycle plastics. Take the task of pulling rare earth elements out of electronic waste. Traditional solvents fall short, especially on efficiency or cost. But certain ionic liquids dissolve the metals straight out of shredded boards without much fuss.
Compatibility: Will It Play Nice with Others?
Chemical compatibility decides whether an ionic liquid can hang out in a specific process—or whether it wrecks the equipment or taints the final product. This mostly comes down to how the ions interact with other materials. Some ionic liquids corrode steel or react with rubber gaskets. Others keep clear of these problems and extend equipment lifespans instead of cutting them short.
In energy storage, picking the right ionic liquid leads to better safety and bigger performance. The wrong liquid, one that chews up lithium or sets off side reactions, spells headaches and short circuits. You see this in flow batteries, where the right pairing keeps the system stable after months of hard work. I’ve worked on teams swapping out legacy solvents for ionic alternatives. The difference, after a few weeks of testing, couldn’t be clearer: the right match keeps everything running, and the wrong one brings the whole project to a crawl with maintenance headaches.
Real-World Choices and Trade-Offs
The story changes once you look at large-scale manufacturing. You don’t just need an ionic liquid that’s soluble or compatible—you need one that’s safe, cheap, and recyclable. Many choices that look great in a flask stumble in the factory. For example, some ionic liquids soak up water from air, which turns mixing into a nightmare and bumps up costs for drying steps. Others stay pure and stable, so they become favorites in fields like pharmaceuticals or surface coatings.
Cost and sustainability figure big in decision-making. Large companies worry about disposal and the potential buildup of tricky byproducts. More chemists in my network keep records on how many recycles a liquid goes through before it breaks down. Choosing an option that recycles ten or twenty times keeps both the science and the business running, which strengthens trust in the technology.
Finding the Best Fit
There’s no single answer for which ionic liquid works best for every job. The right choice comes down to knowing what matters most: rapid solubility for a tough molecule, safe handling alongside sensitive materials, predictable behavior when the heat is on. Getting those answers takes data and experience—plus a healthy respect for the surprises these remarkable liquids still hold.
Understanding the Real Risks Up Close
Everyone hears warnings about chemicals — some seem over the top, others get brushed aside. In my time working around both factories and home projects, I’ve seen what happens when folks ignore those warnings. Scrubbing a surface or mixing compounds without gloves sounds harmless, but irritation or burns show up fast. Once, after thinking little of a “mild” cleaner, I watched a coworker land in the ER with blistered hands. Labels rarely tell the whole story, and people pay the price for shortcuts.
Routes of Exposure People Don’t Always Think About
Hazards often come from unexpected places. Breathing in dust at work can feel like nothing, but lungs do not forgive. Skin contact is equally risky. I learned quickly to respect that, after suffering a rash myself from spilled powder. Some chemicals cause redness on the outside and permanent injury underneath. Eating, drinking, or smoking anywhere near chemical storage brings in another layer of exposure. I’ve seen people do all three, forgetting that powders stick to hands and sneak into food without warning. Even water doesn’t wash everything away.
Acute and Long-Term Problems Go Hand in Hand
Short-term effects show up early and hard: coughing, nausea, dizziness, and skin burns signal the need to stop and step back. Over time, workers may notice headaches or notice they’re short of breath. Years of low-level contact stack up, leading to conditions like asthma or, in the worst cases, cancer. OSHA and the CDC both track these stories, linking repeated chemical exposure to disease. A 2022 report from the National Institute for Occupational Safety and Health detailed rising rates of lung complications tied to workplace dust and chemical aerosols. Numbers like these do not lie.
Who Feels It Worse: Children and the Elderly
Children crawl, touch, and mouth things adults overlook. Low body weight means more exposure, per pound, than grown-ups face. Kids might take in a small amount and suffer big effects. Older people, on the other hand, often process toxins more slowly, so what seems minor to a healthy worker can linger dangerously in their systems. Homes with mixed-age families have to treat these risks seriously — simple steps like locked storage and clear labeling can spare families real harm.
Preventing Trouble Before It Grows
Basic protection works. I keep gloves and masks within reach, and I read every label before starting. Material Safety Data Sheets (MSDS) are not fun reading, but I’ve learned from tough experience that ignoring them invites problems. Ventilation counts for a lot — open windows, exhaust fans, and dust masks keep chemicals out of the lungs and off the skin. Companies have a duty to post warnings and give workers hands-on training. Homeowners can check for safer alternatives or dilute substances before use.
In my view, nothing replaces practical knowledge. Asking questions, double-checking procedures, and treating all unknowns with caution turns routine tasks into safer ones. If in doubt, I always check with a professional or poison hotline. That one phone call can mean a world of difference, for anyone on either end of a chemical hazard.