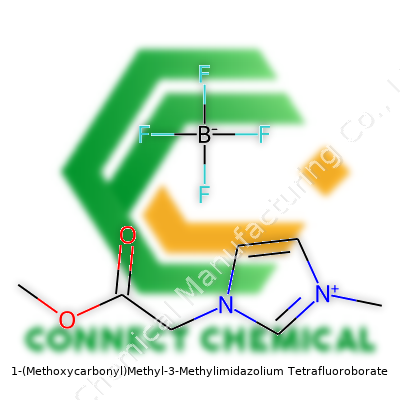
1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate: A Commentary on Progress, Science, and Promise
History of Discovery and Development
Deep in the world of ionic liquids, chemists often chase innovation through endless molecular puzzles. The roots of 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate trace back to decades of trial, error, and curiosity driven by the need for cleaner solvents and safer industrial media. In the 1990s, interest in imidazolium-based salts began to grow as environmental worries mounted over volatile organic compounds. Chemical research journals started reporting syntheses of these compounds, building on a bedrock of earlier work in both organic and inorganic chemistry. The community realized that these substances, with their unique mix of low volatility and wide electrochemical windows, could shift how chemists approach classic separation, extraction, and catalysis. Lab benches saw new recipes and purifications and over time, this particular imidazolium tetrafluoroborate grew in utility thanks to its blend of structural stability and reactivity.
Product Overview
1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate stands out in the family of room-temperature ionic liquids. Commercial catalogs tend to highlight its appeal as both a solvent and a reagent, especially where harsh conditions or conventional substances fall short. The methoxycarbonylmethyl group, paired with the robust imidazolium core, imparts just enough polar character to handle charged intermediates, while the tetrafluoroborate ion brings salt stability without major hygroscopicity. Researchers prize this combination for lab work, as well as in industrial electrochemical setups where voltage stability and ion transport are critical.
Physical and Chemical Properties
This salt pours as a colorless to pale yellow fluid at room temperature, hinting at its low melting behavior. With a density that hovers near 1.2 g/cm³, it provides a heavier medium than most common organics, which can prove useful in separation methods. The compound often resists decomposition under moderate heating, retaining its ability to dissolve both polar and some nonpolar substances. Chemically, it resists hydrolysis better than other ionic liquids with more reactive anions. The imidazolium backbone serves as both a source of mild acidity and as an organic scaffold for further modification, while the tetrafluoroborate counterion brings high ionic conductivity—a property electrochemists love.
Technical Specifications and Labeling
Vials of this compound typically come marked with purity thresholds exceeding 97%, moisture content kept below 1%, and batch lots tested by NMR and mass spectrometry to ensure both quality and safety. Labels carry warnings regarding eye and skin contact, and shipping documents must comply with guidelines for laboratory chemicals, particularly because the tetrafluoroborate group can slowly release fluorine-containing vapors if mishandled. I've seen batch sheets detail beside storage guidelines, as even minor moisture can compromise the compound’s shelf life and some properties important for specific applications.
Preparation Method
Synthetic approaches for 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate usually involve alkylation of 3-methylimidazole with a methoxycarbonylmethyl halide, then metathesis with sodium tetrafluoroborate. The reaction starts in an aprotic polar solvent, sometimes under nitrogen to guard against atmospheric moisture. The mixture stirs at slightly elevated temperature until reactants clear. After solvent removal, the crude imidazolium halide undergoes ion exchange, typically with a slight excess of sodium tetrafluoroborate in water. Purification often involves several rounds of extraction and rotary evaporation, before a final drying step under vacuum. Each synthetic run demands careful control, since trace halides or water leads to side products or spoils subsequent reactions. Teams must record conditions and yields meticulously, since scale-up brings new hurdles in purification and quality control.
Chemical Reactions and Modifications
The imidazolium core welcomes further functionalization. Chemists sometimes append longer alkyl or aryl groups to the methoxycarbonylmethyl arm to modulate viscosity or tune solvent power. The methyl at the 3-position resists most reagents, yielding stability under electrochemical or catalytic conditions. The tetrafluoroborate counterion can be swapped for other anions—hexafluorophosphate, bis(trifluoromethylsulfonyl)imide, or chloride—depending on application needs. Researchers explore these tweaks to match the solvent to new tasks, or to avoid formation of unwanted by-products in tricky syntheses. The parent salt itself sometimes serves as a phase transfer catalyst, shuttling reactants from organic to aqueous media or helping stabilize transition states in multistep organic runs.
Synonyms and Product Names
Across catalogs, this compound’s names multiply. Aside from 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate, you’ll find listings like “MMCIM-BF4,” “Methoxycarbonylmethyl methylimidazolium borate,” or even cryptic alphanumeric codes assigned by supplier databases. Savvy users learn to cross-check product codes and synonyms, since inconsistent labelling sometimes leads to botched orders or cross-contamination risks. The IUPAC style, though wordy, brings clarity; common shorthand takes root in group notes, sketches, and protocols.
Safety and Operational Standards
Lab protocols demand respect for this compound’s reactive nature. Safety goggles and nitrile gloves form the first line of defense, and fume hoods stay busy during both synthesis and handling. Direct skin contact, while less risky than with many chlorinated solvents, can still cause irritation or unexpected allergic reactions. Good ventilation minimizes worries over low-level HF or boron-containing vapors. Waste streams—solvent washes, filtrates, or still bottoms—often count as hazardous, especially if they hold traces of heavier metal salts used in related experiments. Regulators keep a close watch on how these substances enter and exit labs, and proper labeling helps track usage for both worker safety and environmental stewardship.
Application Area
From green catalysis to next-generation batteries, the practical side of 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate stretches wide. Organic labs reach for it during organometallic syntheses and transition-metal-catalyzed coupling reactions, since it rarely interferes with sensitive reactants. Electrochemists design sensors, supercapacitors, and fuel cell components around its ionic conductivity and stability. Process engineers eye it for industrial separations or extraction tasks once dominated by volatile, toxic solvents. Pharmaceutical researchers probe its potential for biocompatible processing, always balancing solvency power against cost and purity. Even in niche research, the compound’s behavior—both as a medium and as a chemical partner—often opens doors to experiments otherwise considered unworkable or dangerous.
Research and Development
My own experience echoes what literature suggests: tweaking the side chains or swapping anions can yield surprising shifts in physical properties or compatibility with diverse chemical systems. Teams at research institutes now run parallel investigations into using imidazolium tetrafluoroborates for carbon capture, biomass conversion, and design of recyclable reaction media. Collaboration between academic chemists and industrial partners has begun to push the boundaries, moving from benchtop curiosity to pilot-scale adoption. Many grant agencies prioritize funding for green chemistry, and these ionic liquids often anchor grant narratives. The impact shows up in new process patents and in a growing number of technical publications delving into structure-activity relationships, lifecycle assessment, and long-term stability under recycled use.
Toxicity Research
Health regulators want real data on long-term exposure risks, especially as usage scales up. Early bioassays hint at low acute toxicity, but chronic studies remain scarce. Typical routes of exposure—spill, inhalation, or oral uptake—rarely mirror actual lab practice, so most hazard analyses focus on accidental releases, environmental persistence, and breakdown in sewage systems. The tetrafluoroborate anion draws attention for its potential to furnish free fluoride under strong acid or high-temperature conditions. Researchers linger over partition coefficients and aquatic toxicity, hunting for red flags before these compounds move beyond lab-scale work. From my own reading, care with waste and obsessive process documentation matter just as much as MSDS compliance if labs want to sidestep regulatory pushback.
Future Prospects
Demand for safer, greener alternatives in mainstream chemistry stimulates efforts to improve both the production and performance of imidazolium-based salts. As companies devote resources toward reducing the cost and environmental footprint of these substances, new methods could cheapen synthesis or boost recyclability. Large grant challenges target carbon-negative chemistry, and these ionic liquids look positioned to play a major role—offering both utility in tough reactions and a path toward cleaner industrial outputs. Some groups also look to fine-tune toxicology and waste management strategies, aiming to meet stricter government standards without sacrificing real-world function. Open-ended collaboration between academic, commercial, and regulatory partners should accelerate innovation, so future students and industry chemists inherit better, safer, and more versatile chemical tools.
Understanding the Compound
Anyone working in a modern chemistry lab knows things move fast. Every so often, a new tool shows up, bringing fresh opportunities for making difficult processes easier or greener. 1-(Methoxycarbonyl)Methyl-3-Methylimidazolium Tetrafluoroborate—let’s call it MMCIM BF4—shows up more and more in both academic research and industry settings. This ionic liquid has people paying attention for good reasons.
Why Chemists Reach for Ionic Liquids Like MMCIM BF4
In chemistry, solvents play a big role. Traditional solvents often carry health and environmental risks. Ionic liquids such as MMCIM BF4 break the mold. They usually don’t evaporate at room temperature. This means less toxic fumes, less fire risk, and fewer regulatory headaches. MMCIM BF4 stands out for its stability and unique mix of properties: it dissolves a wide variety of organic and inorganic compounds, doesn’t flinch in the face of moisture, and keeps steady under heat.
The main draw for this molecule comes in organic synthesis. Reactions that used to require harsh or volatile chemicals can now happen in milder, safer conditions. I’ve noticed chemists using MMCIM BF4 during cycloaddition or alkylation steps. These reactions demand both high selectivity and a gentle environment. The tetrafluoroborate part lends extra stability, keeping side reactions at bay. Some researchers even recover and reuse their ionic liquid, saving money and respecting strict waste guidelines.
Green Chemistry: Reducing the Chemical Footprint
MMCIM BF4 fits right into the movement toward sustainable chemistry. Many companies and universities pursue “greener” methods not only to meet new regulations, but to protect workers and communities. The low volatility of this compound means labs release fewer pollutants. Unlike common solvents such as dichloromethane, MMCIM BF4 sticks around. Fewer environmental issues crop up, and disposal costs go down.
It also supports catalyst recycling. Catalysts tend to be costly, often made from rare metals. In my own time managing a pilot lab, we always struggled to keep platinum catalysts working efficiently. With MMCIM BF4, catalysts sometimes stay active longer, and recoveries become easier. The ionic liquid forms a phase with the catalyst, allowing product extraction without wasteful washing steps.
Challenges Still on the Table
There’s always the flip side. Some ionic liquids raise toxicity questions. Wastewater can turn into a trouble spot, even with more stability. For MMCIM BF4, disposal does not need massive changes to infrastructure, but thoughtful handling still matters. People keep a close eye on the long-term safety profile of these novel solvents.
Production costs set a limit to broad adoption. Large-scale manufacturing needs cheap, safe materials—especially true for pharmaceutical and agrochemical plants. MMCIM BF4 still costs more than old, established solvents, but interest continues to grow as its price comes down.
The Road Ahead
MMCIM BF4 gives chemists a tool for safer and smarter synthesis. Early adopters lead the way, driving process innovation and supporting stronger environmental standards. Labs that value safety, efficiency, and green chemistry will likely keep this ionic liquid close at hand. Careful research on its safety and affordable synthesis should help more chemists switch over, pushing the industry toward safer science.
Keeping Quality Intact
Proper storage transforms a product’s shelf life and the safety of those who use it. Over the years, I’ve seen what ignoring basic storage advice can do—mold in a packet of flour, unstable medication, spoiled pet food. Products aren’t just made to sit anywhere. Each label hinting at a storage suggestion tells its own story, often born out of years of testing and more than a few hard-learned lessons.
Why Storage Changes Everything
Let’s say we’re talking about a food product—or any consumable. Warmth and moisture creep in, bacteria and fungus find a home, and pretty soon, what looked harmless can cause a trip to the doctor. With something like medication, it gets a little scarier. Chemical breakdown can strip it of power or make it unpredictable. Companies stake their reputations on keeping things safe, but they rely on everyone in the chain to respect what storage conditions mean.
What Temperature Means in Real Life
Room temperature—a phrase that shows up everywhere—usually means around 20 to 25 degrees Celsius. In the past, I stored some herbal supplements on a sunny kitchen windowsill. By week three, colors faded, and the bottles didn’t quite smell the same. UV rays and fluctuating heat are real threats that sneak up quietly. Cold storage doesn’t just preserve freshness. It slows the breakdown of vitamins, stops oils from going rancid, and buys companies and customers alike more time.
Humidity Is Half the Battle
Moisture doesn’t just shape the texture of crackers or cookies. Once humidity climbs, products start to sweat, clump, or breed new life forms in what should’ve been a safe package. I remember an expensive whey protein that turned into a rock-solid lump after a few summer days in a damp cupboard. No amount of breaking it apart fixed the damage—or stopped the odd aftertaste.
The Power of Containers
It’s tempting to rip open a bag for easy access or pour contents into a random jar. The original packaging often does more than look nice on a shelf. Foil wraps, desiccant packets, tightly sealed lids—a lot of time goes into designing protection against air, light, and bugs. Missing this step has cost me more money, and plenty of frustration, than I’d like to admit.
Shortcuts Aren’t Worth It
People sometimes tuck products under sinks, on top of fridges, or near stoves out of habit. The heat from these spots builds up fast. Even storing near a bathroom exposes things to regular moisture. It seems harmless, up until the product starts changing color, develops new smells, or doesn’t work as promised.
How to Get it Right
For anyone lost in a sea of labels, start simple. Check for icons or instructions on the package. If a space stays cool, out of the sun, and free from wild swings in humidity, it’s usually safe. Pharmacies and grocery stores often do walkthroughs for staff to teach this stuff—what goes in the fridge, what stays sealed, where to stack boxes so air flows around them. Taking those few extra seconds to follow directions keeps the contents safe, and saves money, time, and health in the long run.
What Makes This Chemical Unique?
1-(Methoxycarbonyl)methyl-3-methylimidazolium tetrafluoroborate sounds complicated, but it belongs to a class of chemicals called ionic liquids. These compounds catch the attention of scientists and industry because they often have low volatility compared to more traditional solvents. That means you won’t see much vapor coming off them, and they aren’t likely to explode like gasoline or alcohol. Many labs use substances like these for organic synthesis, extractions, or as electrolytes in batteries. The hype about “greener” chemistry sometimes centers on these ionic liquids, promising more safety—at least compared to classic solvents. Still, safe on paper doesn't always mean safe in your hands.
Looking at Toxicity: What’s Known?
If you go looking for detailed toxicity data on this particular compound, you won’t find much outside of academic sources. The lack of direct evidence isn’t a guarantee of safety. Fluorinated compounds can have tricky behavior. Tetrafluoroborate, the anion part of this molecule, isn’t something you want to ingest or get all over your skin. Imidazolium ionic liquids don’t send out obvious warning bells, but that’s partly because they haven’t been in use for many decades in bulk—and chronic health effects often take years to surface.
Occupational health guides—everything from the European Chemicals Agency (ECHA) to NIOSH in the United States—tend to flag general risks for related ionic liquids. These risks include irritation of skin, eyes, and the respiratory tract. Inhalation could irritate mucous membranes and lungs if you don’t have proper ventilation. Some research points out that methylimidazolium compounds can disrupt enzymes in living organisms or harm aquatic life if spilled. That’s not theoretical. Studies with zebrafish and daphnia show stress, slowed development, and sometimes lethality after exposure. And if a chemical can mess with enzymes in fish or shrimp, safe bets should involve gloves and goggles at least.
Environmental Footprint and Lasting Impact
You hear the phrase “green chemistry” tied to ionic liquids in journal articles, but greenness depends on the lifecycle. The breakdown products of tetrafluoroborate salts can include boron- or fluorine-containing compounds, which are not always innocent. Some persist in soil and water. As a part-time science educator, I’ve seen examples where good intentions—like swapping out volatile solvents for ionic liquids—just replaced one set of problems with another. Toothpaste once used triclosan to kill bacteria, until enough research linked it to hormone disruption and river pollution, and the world had to rethink. The same vigilance holds with new lab chemicals.
Reducing Hazards—A Hands-On Approach
For any lab or production process using this compound, safety steps start with personal protective equipment and closed handling. If your workflow puts you in contact with powders, consult the latest Safety Data Sheet (SDS) and ask questions if any reference is vague. Disposal requires care—down the drain doesn’t cut it. Waste from ionic liquids often demands specialized chemical waste collection firms, with traceability in paperwork.
Substitution remains the clearest way to avoid unknown risks. If a less persistent or more thoroughly studied solvent can do the same job in your process, switching over may prevent headaches. Teaching young scientists, I’ve learned the new isn’t always safer; sometimes it just takes longer for the risks to come clear.
Key Takeaways for Everyday Handling
Work in well-ventilated spaces. Use proper disposal pathways. Push for transparency from suppliers about toxicity and long-term effects. Never fall for “green” labels without reading the fine print. In chemical safety, the human element—steady, thoughtful, and a bit skeptical—makes more difference than any single new formula.
Understanding Chemical Formulas
A chemical formula tells a story about a molecule’s structure. It shows which elements form the compound and the proportion of each. For example, water’s formula is H2O. Two hydrogen atoms connect to one oxygen atom. This type of notation strips a complicated substance down to its building blocks, making it possible to predict how molecules react, interact, and support life or industry.
Understanding formulas may seem like basic science, but it plays a major role far beyond the classroom. Take baking soda (NaHCO3). Knowing what’s in it keeps people safe in the kitchen and lets manufacturers label food properly. It matters in farming too. Fertilizers must include a clear breakdown, both for compliance and so farmers avoid polluting soil or water with excess chemicals. This shows that chemical formulas allow for transparency—from food labels to environmental regulation.
Molecular Weight: More Than Just a Math Problem
Molecular weight, or molecular mass, carries heavy significance in the real world. It’s the sum of the atomic masses of all atoms in a molecule. Let’s look at glucose (C6H12O6). By adding up the atomic weights (carbon: 12, hydrogen: 1, oxygen: 16), you see glucose weighs 180 grams per mole. It sounds dry, but this number drives practical decisions every day.
In pharmacology, dosing cannot be left to guesswork. Each pill or injection depends on the molecular weight of a drug to make sure patients get safe amounts. I’ve met pharmacists who triple-check their math—one decimal point can be the difference between a cure and a complication. Similar math applies in labs and factories, where recipes call for exact ratios to ensure a reaction works, whether brewing beer or purifying water.
Why Accuracy and Understanding Matter
Getting chemical formulas and molecular weights right is not just for scientists. Misreading a label or miscalculating a dose can lead to real harm. The Texas City disaster in 1947 comes to mind. Mistaking the properties of ammonium nitrate contributed to one of the worst industrial accidents in U.S. history, highlighting what can happen when chemistry gets overlooked.
For anyone handling chemicals—at home or in a plant—understanding what those formulas mean reduces risk. The rise of accessible information has made it easier to double-check the basics before mixing substances, dissolving pills, or engineering new materials. There is value in fostering curiosity and a willingness to check facts, not just for specialists, but for anyone who touches chemistry.
Building Better Habits for Chemistry Literacy
Education forms the backbone of chemical safety and progress. More interactive science lessons and public outreach can make chemistry less intimidating. Open data and clear labels help people make informed decisions, pushing back against accidents or misuse.
Tools like digital molecular calculators and mobile apps lower the barrier for precision. Students, parents, and professionals can reach for reliable references instead of trusting outdated notes or vague memories. The more chemistry becomes part of everyday life, the safer and more creative people become in kitchens, garages, gardens, and labs.
Smarter Choices Through Chemistry
Numbers and codes behind chemical substances tell more than just what makes up a product—they guide actions, safety, and creativity. Better chemistry literacy leads to safer handling, smarter choices, and new possibilities both at home and in industry.
Real Value in Ionic Liquids
Ionic liquids catch a lot of attention in research circles because of their special traits. Unlike everyday solvents, these don’t easily evaporate, and they keep their cool under heat and pressure. This sort of resilience fits industries looking for ways to do more with less pollution. Labs have shown that with the right ionic liquids, industrial reactions can hit high efficiency targets at lower temperatures, saving energy and sometimes giving purer results.
That said, not every substance or compound joins the party so easily. The choice for use in ionic liquids comes down to chemical compatibility and what exactly you want to achieve. Certain salts and organics blend with ionic liquids and keep stable, while others break down or fight the process. In my experience on projects looking for green alternatives in chemical processing, we found that even tiny tweaks in structure could make or break performance. Something that works as a solvent or catalyst in water often acts differently in an ionic environment, or might not mix at all. So, it takes hands-on testing before full-scale shifts.
Hopes and Hurdles for Catalyst Use
Catalysts are like the unsung heroes in chemistry—they drive reactions fast and cleanly, making all sorts of things possible in food, fuel, plastics, and pharma. People keep looking for catalysts that work better or last longer, especially under greener, milder conditions. Ionic liquids sometimes help these reactions along by giving a stable, polar environment where traditional catalysts may fail. Certain metal ions, acids, and even enzymes have worked in ionic liquid media in real-world demonstrations.
Among challenges, cost of sourcing or preparing these liquids can get high, often outweighing the greener benefits unless the process gets used at big scales or recycles the liquid. Add to this the trouble of cleaning out the final product—removing every trace sometimes calls for extra steps, making the whole thing less appealing for commercial use. Regulatory agencies watch carefully, too, because not every ionic liquid used in a lab is ready for food tech or pharma.
Why This Matters for Innovation
We need better routes to cleaner, more efficient chemistry. From agriculture to plastics recycling, slim margins and stricter regulations push companies to look for smarter ways to keep processes safe and sustainable. Even if today’s ionic liquid options only fit niche applications, breakthroughs can spread fast. A famous case is the BASF process for cellulose dissolution, which relies on ionic liquids, making some formerly tough materials accessible as sustainable feedstocks.
Fact-based decisions can change the landscape. In a project where we trialed alternative solvents for pharmaceutical synthesis, the ability to reuse ionic liquid for several batches without loss in yield cut down waste and saved money in the long run. Careful upfront investment paid off as stricter wastewater rules came into force. This real saving shows why careful pilot studies, backed by strong analytical methods, should guide adoption.
Next Steps Toward Practical Solutions
Teams serious about using new solvents or catalysts need to get practical. Screen each option for stability, ease of recovery, and long-term safety. Focus on collaboration between academic researchers and industry process engineers. Push for clear documentation of successes and failures to stop others from going down dead ends. Once scalability and safety align, regulatory approval follows. Only then do these advances shape up as real solutions in everyday chemical problems.