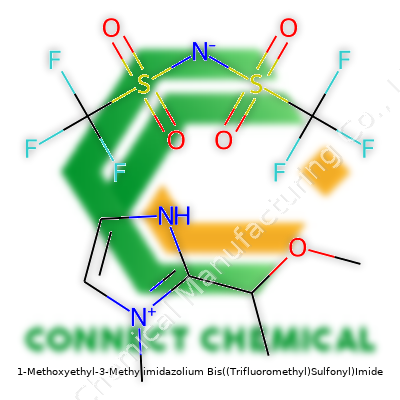
1-Methoxyethyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide: A Down-to-Earth Look
Historical Development
The journey of modern ionic liquids starts with the search for alternatives to traditional solvents. Early researchers saw the potential in imidazolium-based salts, recognizing their liquid state at room temperature and unique solvent properties. In the 1990s, chemists ventured into new alkyl chain substitutions to improve physical stability and targeting green chemistry goals. The discovery of 1-Methoxyethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide comes from this small but passionate group of researchers on a mission to replace volatile and toxic solvents used in industry. Over several decades, stepwise advances in synthetic organic chemistry allowed the design and scale-up of this salt, cultivated by the shared vision of safer, more reliable materials for chemistry, materials science, and energy storage.
Product Overview
At its essence, 1-Methoxyethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide is a room-temperature ionic liquid. Its name points to its structure—a positively charged imidazolium core, an ether-functionalized side chain, and a robust bis(trifluoromethanesulfonyl)imide (NTf2) anion. Industries prefer this salt for its low vapor pressure, resilience in both air and moisture, and ability to dissolve a broad range of chemicals. Its colorless-to-pale yellow appearance underscores its purity and consistency, while its almost non-existent odor offers advantages for lab and manufacturing settings. Companies sell it as a mobile, viscous liquid in sealed, moisture-resistant containers, often displaying impressive shelf stability when stored carefully.
Physical & Chemical Properties
A look at physical and chemical benchmarks reveals several talking points. The liquid’s viscosity usually runs higher than water or acetone, hovering between several tens to hundreds of centipoise at room temperature, depending on purity and storage time. Scientists routinely measure conductivity values between 2 to 4 mS/cm, making it attractive for electrochemical applications. Its melting point sits comfortably below room temperature, often below −20°C, which frees up handling and transfer in labs. The NTf2 anion lends chemical and thermal robustness, keeping the liquid stable well beyond 200°C before visible decomposition. Unusually low volatility gives it a leg up in workplace safety and environmental protection. Its solubility profile stands out—mixing with many organic compounds, polar plastics, and even some inorganic salts, yet rarely dissolving in water thanks to hydrophobic tails and strong ionic bonds.
Technical Specifications & Labeling
Producers grade this compound at 97% or above for most research and industrial batches, flagging water levels often under 0.2% by Karl Fischer titration. Key specifications include density (typically 1.38-1.44 g/mL at 25°C), refractive index (about 1.435), and electrical conductivity, each measured with sharp monitoring to track contamination or decomposition from extended storage. Labels highlight the chemical formula, lot number, storage temperature, and hazard pictograms, alerting users to skin or eye contact risks. Material Safety Data Sheets recommend using it within a year, offering best-before dates and details on disposal routes under current hazardous waste regulations.
Preparation Method
Synthesizing this ionic liquid draws from classic organic methodologies and modern energy-efficient improvements. Chemists start by quaternizing 1-methoxyethylimidazole with methylating agents under nitrogen, forming a methylimidazolium cation. The next step swaps a halide for NTf2 anion using lithium bis(trifluoromethanesulfonyl)imide in a two-phase extraction, taking advantage of its insolubility in water. After separation, thorough washes remove trace halides and byproducts, and filtration through activated carbon or silica gel eliminates color-producing contaminants. A final drying phase under vacuum assures minimal water content. In practice, yields above 85% are common, with proper glovebox or Schlenk line techniques limiting exposure to atmospheric moisture.
Chemical Reactions & Modifications
Not many chemicals match this compound’s resistance to acids, bases, and high temperatures. It absorbs strong acids or bases without rapid breakdown, only starting to show signs of instability in the presence of strong Lewis acids or long-term ultraviolet radiation. The NTf2 anion keeps thermal decomposition in check. Where modifications do happen, researchers have experimented by switching out ether or alkyl groups on the imidazolium ring, yielding new ionic liquids with subtle shifts in solubility, viscosity, and hydrophobic character. This flexibility supports custom formulations for energizing everything from catalysis to surface modification.
Synonyms & Product Names
The same molecule travels under several titles in academic and industrial circles—common ones include [1-methoxyethyl-3-methylimidazolium][NTf2], [C1OC2mim][NTf2], or simply CMMIm NTf2. Major suppliers add their own product identities and catalog numbers, but the backbone remains the same. Recognizing these aliases avoids confusion when sorting through chemical catalogs or research articles.
Safety & Operational Standards
Handling this ionic liquid feels less hazardous than some volatile organic solvents, but care is still crucial. Contact with eyes or skin provokes irritation, so gloves and eye protection become standard fare in labs. Accidental ingestion or inhalation raises health concerns due to the imidazolium backbone and possible traces of starting materials. Once spilled, clean-up practices follow strict absorbent use and proper disposal. Laboratories and factories focus on minimizing waste generation and sealing used samples tightly. Regular air monitoring, material tracking, and responsible disposal reinforce safety protocols established through years of chemical handling experience.
Application Area
The draw of 1-methoxyethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide rests in its adaptability. I’ve seen this ionic liquid at work in catalysis laboratories, where it dissolves reactants that refuse to cooperate in standard solvents. Fuel cell technologists tap its ion transport characteristics to improve polymer electrolyte membranes. Batteries benefit from this salt’s broad electrochemical window and thermal stability, supporting next-gen lithium-ion and flow battery systems. In synthetic chemistry, it makes stubborn extractions and separations routine. Other groups deploy it for CO2 capture and green chemistry innovations, sidestepping volatile and flammable alternatives. Its uptake continues as environmental and economic pressures force the rethink of traditional chemical processes.
Research & Development
Academic and corporate teams push at the boundaries of this molecule’s usefulness each year. Within my own professional network, research pivots around ionic conductivity, solvent tolerance, and functional group compatibility. Leading labs log data on viscosity, conductivity, and lifetime under real-world stress to feed computational models, bridging the gap between theory and field applications. Several patents spotlight custom blends that match specific battery chemistries or environmental remediation projects. This continued R&D sprint promises smarter, more sustainable ways to deploy these liquids without sacrificing efficiency or safety.
Toxicity Research
Toxicologists cast a critical eye at the rise of almost any new solvent. Bioassays, aquatic life studies, and human exposure modeling dominate research papers about this salt. Several findings point to moderate toxicity if mishandled — eye and skin irritation lead the list, but no acute life-threatening effects show up at expected workplace exposure levels. Yet, researchers keep one foot on the brake because breakdown products or traces from synthesis could pose risks over long-term exposure. Regulatory agencies and safety professionals call for individual risk assessments and greener molecular designs, not just in the lab, but across industry.
Future Prospects
Looking ahead, 1-methoxyethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide sits at an interesting crossroads. Industrial and research cycles now reward substances that lower pollution, resist fires and help unlock renewable energy. This salt fits some of these categories, yet questions around cost, raw material sourcing, and full-cycle toxicity linger. Chemists and engineers will likely keep tuning the molecule’s recipe for easier recycling and lower toxicity. I see a strong chance for new applications in carbon capture, advanced separations, and battery innovations as breakthroughs build on both data and practical handling experience.
Understanding the Role of Advanced Ionic Liquids
Some chemicals tend to stay under the radar, even though they carry big responsibilities in research and industry. One of those is 1-Methoxyethyl-3-Methylimidazolium Bis((Trifluoromethyl)Sulfonyl)Imide. This name doesn’t roll off the tongue, and it’s not something you find in household products. Despite that, its use makes a difference in labs, battery development, and new methods for recycling materials.
Pushing the Boundaries of Green Chemistry
This compound falls into the category of ionic liquids. Unlike typical solvents that evaporate easily and create harmful fumes, ionic liquids hold onto their structure and rarely evaporate at room temperature. That makes them intriguing for chemists who want to minimize the environmental impact of their work. Over the last decade, ionic liquids have become essential in the hunt for safer, greener alternatives to volatile organic solvents. I’ve seen how the shift toward greener solvents not only changes lab safety but also lowers cleanup costs and keeps chemical waste down.
It’s no secret that traditional solvents come with health risks and fire hazards. Swapping in ionic liquids such as this one gives researchers more breathing room—literally and figuratively. The lack of fumes and lower toxicity matter in both personal experience and published research. It’s become easier for universities and large companies to comply with stricter environmental and workplace safety rules thanks to new solvent choices.
Breaking Open New Paths in Battery Tech and Extraction
As a chemist, I’ve watched teams try to make batteries safer and more efficient. The electrolyte, that often-overlooked part of the battery, can decide how long a battery lasts. Many standard batteries use solvents that degrade under high voltage, which shortens battery lifespan and, in extreme cases, sparks safety problems. Ionic liquids like this one can stand up to those high voltages, acting as more stable electrolytes. Research shows they don’t catch fire as easily and maintain their structure after cycling through hundreds of charge and discharge cycles.
Aside from batteries, this compound works well in metal extraction and separation processes. Metal-rich ores contain valuable elements mixed up with plenty of unwanted material. Traditional methods use aggressive chemicals and generate toxic runoff. Researchers are drawn to ionic liquids because of their ability to dissolve or transport specific metals, offering selective extraction with less environmental fallout. There’s a clear trend toward using ionic liquids as part of closed-loop systems, which keep chemicals in circulation and protect waterways.
What’s the Catch? Cost, Knowledge, and Scale
Not everything is perfect. While ionic liquids like this compound shine on paper, the price tag holds back wider adoption. Synthesis costs more compared to old-school solvents, which keeps big industries cautious. People also want to see long-term data on performance, safety, and contamination before overhauling whole processes.
I’ve seen teams get creative, teaming up with academic groups and government agencies to unlock better synthesis methods. Publishing open data about ionic liquids’ performance across a range of applications helps more eyes spot possible issues early. With ongoing funding and curiosity, better solutions should come out of the pipeline. The next chapter of clean chemistry and safer materials is already being written in specialized labs, and this compound gets plenty of attention from those ready to push boundaries.
Understanding Ionic Liquids Beyond the Lab
Years spent working with unconventional solvents have shown me how ionic liquids keep grabbing the attention of chemists, engineers, and even folks trying to clean up industrial sites. Unlike what you see pouring out of the bottle in high school chem class, these liquids break the mold. Many people see them as just another chemical, but if you've ever handled one, the experience challenges a lot of expectations.
Why Low Melting Point Matters
One standout quality is their low melting point. Traditional salts such as sodium chloride keep their solid form until the heat cranks up past 800 degrees Celsius. With many ionic liquids, room temperature is enough to get them flowing. This comes from their unique structure: big, bulky organic cations matched with various anions. These awkwardly shaped ions can’t pack together in neat crystals, so the substance stays liquid at far lower temperatures. There's no denying how this helps — pour, pump, or spread ionic liquid wherever you need without elaborate heating systems.
Evaporation Isn't a Problem
Standard solvents like acetone or hexane evaporate as soon as you take the top off the bottle, and fumes fill the air. Ionic liquids go the opposite way. Their negligible vapor pressure keeps the liquid from vanishing into the atmosphere, even on a hot day. This cuts down on waste and limits exposure to hazardous volatiles. For workers concerned with occupational safety or for environmental chemists tracking air emissions, this property frequently tips the scales toward ionic liquid solutions.
Handling Density and Viscosity
Pick up a flask of ionic liquid and you'll notice the weight. High densities are normal, often ranging from 1.1 to 1.6 grams per cubic centimeter. Lab techs and process engineers feel the difference, especially compared to oil or most organic solvents. On the downside, some types can feel like syrup — viscosity changes depending on temperature and the specific ions present. This creates both challenges and opportunities: slow mixing might frustrate, but that same thickness can help trap reagents right where a reaction needs to happen.
Why Their Chemical Stability Grabs Attention
Stubbornness in the face of heat, oxygen, or water isn’t something you get with every solvent. Ionic liquids push back against decomposition even at temperatures between 200 and 400 degrees Celsius. This unlocks all sorts of reactions you would never attempt in standard media. It also means fewer breakdown products to clean out during downstream processing. Over the past decade, I've seen this trait open the door for greener catalytic reactions and electrochemistry projects, especially where water or acid-sensitive reagents play a role.
Polarity and Solubility: Designing for Specific Jobs
Some people call ionic liquids “designer solvents” for good reason. By mixing and matching ions, chemists tune polarity and solubility to fit a purpose. Water-miscible versions dissolve salts or polar compounds with ease, while others lock out moisture and pull in organic molecules. This customization lets people extract metals, run enzyme reactions, or develop new battery electrolytes — the properties shift right alongside the recipe. Tailoring the mix supports sustainability in most current designs, giving rise to recyclable, reusable solvents that sidestep pollution from common alternatives.
Tackling the Roadblocks
Before calling these liquids a panacea, the field has to face concerns over toxicity and biodegradability. Early versions did not always play nice with living things or break down fast in the wild. Good news — new families show real improvement, but the search for safer, cleaner options never ends. Transparency about life cycle impacts matters for everyone using them outside the research bench.
Push for Smarter Solutions
Every year, more industries — from pharmaceuticals to renewable energy — invest in better ionic liquids. Success means marrying cutting-edge research with plain accountability about long-term impacts. Smarter design, honest data sharing, and tighter recycling help turn this odd class of liquids from curious lab sample into mainstream toolbox essential.
Looking Past the Label
Plenty of everyday products come with warning labels or safety data sheets. That’s a good thing, but you won’t always get the full story from small print. Companies have requirements to share the bad stuff, but risks can still slip through. As someone who has worked with industrial supplies for over a decade, I’ve seen folks let their guard down around products just because they’re familiar or look harmless. The truth: harmless labels don’t always mean the substance won’t cause you grief.
The Real Risks Hiding in Plain Sight
Let’s talk about cleaning sprays, adhesives, and DIY materials. A household cleaner with bleach or ammonia doesn’t sound too dangerous when you’re shopping. Start mixing cleaners or use them in a poorly ventilated space, and suddenly you’re breathing in gasses that irritate lungs or worse. Paint thinners and glues do more than stink up a room—they release volatile organic compounds (VOCs) that have been linked to headaches, dizziness, and long-term nerve damage. Even products marked “green” or “eco-friendly” often contain chemicals that haven’t been studied enough for honest safety claims.
We’re only scratching the surface. Flame retardants in furniture linger in dust then end up in our bodies. Plastics leach chemicals like BPA and phthalates, which can affect hormones. Pesticides don’t always stay outdoors—they get tracked in on shoes and stick to surfaces where kids play. These chemicals aren’t only someone else’s problem. Nearly everyone interacts with them daily, even if they don’t recognize the names on the ingredients list.
Why Awareness Matters
My own experience with a persistent cough led me to question products around my home. After switching to safer options and paying attention to home ventilation, the cough faded away. The lesson is clear: just because a product hasn’t sent you to the ER doesn’t mean it belongs in your air, water, or skin. The Environmental Protection Agency (EPA) has shown some chemicals build up over time, contributing to everything from allergies to higher cancer risk. This isn’t just worry-mongering; peer-reviewed studies back the connection between some toxins and chronic health problems.
Learning to Read Between the Lines
A few steps go a long way. Look for full ingredient lists, not just the word “fragrance” or vague descriptions. Check regulatory databases—the EPA and European Union offer public tools making it easy to look up chemical health ratings. Occupational Safety and Health Administration (OSHA) Material Safety Data Sheets, or SDS sheets, reveal what kind of gloves, mask, or ventilation a product expects you to use. Ignore the marketing spin—dig deeper. Consumer Reports and Environmental Working Group publish guides with grades on common products.
Better Choices, Safer Spaces
No one expects perfection, but more information leads to better decisions. Choosing products with simple, limited ingredients or using physical cleaning methods like steam or microfiber cuts down on exposure. Good ventilation and hand-washing help stop accidental ingestion or contact. When in doubt, reach out to poison control centers or toxicology organizations—they’ll talk in plain language without the industry fluff. Products may not look hazardous, but your health doesn’t need to play guessing games. Stay curious, ask questions, and don’t settle for half-truths when safety is on the line.
Why Storage Conditions Matter
Ask anyone who’s handled chemicals long enough – mistakes with storage have ripple effects that reach beyond cost and inconvenience. Stories float around university labs about containers sweating, crystals plugging lids, fumes setting off alarms or corroding shelf labels. A misplaced drum in a humid room can become a ticking hazard. Following the right storage approach, backed by reliable evidence, saves more than just dollars – it keeps people safe and protects the integrity of the chemical itself.
Temperature and Humidity: Not Just Numbers
Chemicals don’t take well to extremes. Take peroxide: let the room get too warm or allow sunlight to hit the bottle, and stability drops fast. Once, I stored methyl ethyl ketone in a space that swung between cold nights and scorching days. The next time I opened the can, fumes hit me like a wall – solvent loss had kicked in, making the mixture a safety nightmare.
Maximum shelf life and stable performance often mean aiming for a cool, dry spot. Research from the Occupational Safety and Health Administration (OSHA) and the National Fire Protection Association (NFPA) points to a sweet spot around 20-25°C and below 60% relative humidity for organic compounds. No, you don’t need an industrial chiller for every bottle. Just avoid attics, sun-facing windowsills, or anywhere prone to heat spikes. Even common sodium hypochlorite, the basic bleach, degrades fast if it sits in the heat – by six months you’re left with a fraction of the strength you started with.
Segregation Beats Chaos
Too many places try to cram everything onto one shelf for convenience. Strong acids, bases, solvents, and oxidizers each demand their own zone. In one warehouse I worked, a simple shelf-mapping exercise revealed an oxidizer next to a bin of oily rags. That’s a recipe for disaster. The Centers for Disease Control and Prevention (CDC) recommends storage by hazard class. If a spill happens, you want to avoid creating new problems. Think acids away from bases, combustible materials far from oxidizers, flammables in fire-rated cabinets. It isn’t overkill. Chemical safety data sheets (SDS) give clear advice for each material for a reason.
Packaging and Ventilation: Not Just Technicalities
Labels wash out, lids warp, cartons break down when the wrong containers hang out in the wrong environment. I’ve seen vinyl gloves stick together and glass vials sweat if they stay too long in damp conditions. Even the best plastic bottles become brittle or degrade if exposed to UV or ozone. Keep original packaging intact, place containers away from direct sun, and store liquids below eye level to reduce splash risk. The American Chemical Society (ACS) recommends using corrosion-resistant trays as secondary containment for acids or bases – a tip that’s saved more than one workbench from nasty etching.
Decent airflow keeps fumes low. Flammable chemicals, in particular, deserve well-ventilated lockers with spark-proof vents. One study from the National Institute for Occupational Safety and Health (NIOSH) highlights that chronic exposure to low-level vapors, often invisible and odorless, can have real health impacts over time, even if nothing dramatic happens in a single shift.
Better Habits, Fewer Accidents
Chemical storage isn’t something to set and forget. Regular checks expose issues early: leaky lids, crusty containers, expired stock. Nobody looks forward to audits, but a half-hour audit and a marker can turn a hazardous shelf into a safe one. Involve staff, hold quick reviews, and toss expired or damaged containers before they come back to haunt you. Good storage conditions respect both the science behind the chemicals and the people around them.
The Essential Nature of SDS in Chemical Handling
Safety never takes a back seat in a lab or manufacturing space. Anyone working around chemicals knows the value of a reliable Safety Data Sheet—or SDS—because it’s not just about avoiding a mishap. It’s about trust in your process, protecting your health, and respecting the environment. I’ve spent enough time in research buildings to learn that guessing about a substance’s hazards makes you a danger not just to yourself but to everyone around you.
1-Methoxyethyl-3-methylimidazolium bis((trifluoromethyl)sulfonyl)imide sounds like a mouthful, but it’s part of the ionic liquids family. These chemicals offer tons of benefits for researchers and industry—great thermal stability, low vapor pressure, and the ability to dissolve wide-ranging substances. Still, their unfamiliar structures mean many users may not know how to handle or store them off the top of their heads. That’s exactly why finding the right SDS matters.
How to Track Down the Safety Data Sheet
Start with your supplier. Major chemical distributors like Sigma-Aldrich, Alfa Aesar, and TCI America build strong reputations on reliable sourcing and complete documentation. Their websites put searchable databases front and center, where you can grab an SDS in just a couple clicks. Type the product name into the bar and download the latest version. You may notice regional variants, so always check that your SDS matches the regulations for your country.
If your chemical comes from a smaller supplier, check for a product code or batch number on the packaging. This can help customer service reps direct you to the exact sheet you need. Don’t toss that original shipment paperwork—in tight situations, technical support teams can use order info to trace the document much faster.
The Paper Trail: Regulation and Responsibility
Current OSHA guidance tells employers to keep an SDS accessible for every hazardous substance on-site. That’s not just paperwork for the sake of it. Inspectors actually look for this during audits, and missing documents invite fines or shutdowns in a hurry. European labs fall under REACH requirements (Registration, Evaluation, Authorisation and Restriction of Chemicals), which push for the same strict traceability.
Running a tight ship isn’t just about meeting regulations. Hospitals don’t function if staff don’t know how to deal with exposure, spill, or fire scenarios. Lab managers who routinely train staff on chemical hazards—not just for the glitzy stuff like sodium cyanide, but even less dramatic compounds like this ionic liquid—help prevent almost every problem I’ve ever seen. An SDS is only useful if everyone actually reads it.
Barriers and Solutions
Some chemicals, including newer or custom-formulated ionic liquids, don’t show up in search results right away. In those cases, persistence matters. Reach out directly to the manufacturer. If documentation is missing, insist on it. Regulators are clear: every chemical, no matter how niche, gets its sheet. Companies that ignore this expose themselves and their customers to risk.
Colleagues sometimes trade SDS files over email or internal databases. That works in a pinch, but keep in mind SDS revisions do come out as safety standards shift. Stay current and don’t rely on decade-old paperwork. The effort you spend on up-to-date protocols pays off in peace of mind and safer routines.
Building a habit of searching, downloading, and actually reading an SDS keeps teams safe. For me, there’s no shortcut to safety. Reliable access comes from knowing your suppliers, building routines for documentation, and not being afraid to demand what’s needed from your vendors. That’s how real safety gets done.