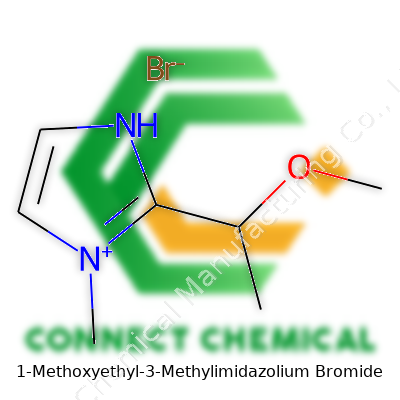
1-Methoxyethyl-3-Methylimidazolium Bromide: An In-Depth Commentary
Historical Development
Over the last few decades, ionic liquids have attracted the attention of chemists searching for cleaner solutions in synthesis and separation. Among them, 1-methoxyethyl-3-methylimidazolium bromide (abbreviated as [MOE-MIM]Br) traces its roots to the steadily growing family of imidazolium-based ionic liquids. Synthetic routes emerged from the classic methylation of imidazoles, but folks at research labs quickly found that adding a methoxyethyl group to the imidazolium core changed the game. Laboratories in Europe and Asia contributed data around the late 1990s and early 2000s, as researchers realized these tweaks made the material more soluble in organic solvents and water, pointing toward a host of new uses.
Product Overview
[MOE-MIM]Br is no household name, but inside research and pilot-scale factories, bottles of this substance sit on benches as pale white or off-white crystalline solids. The compound carries rather low volatility, an advantage that makes it less likely to vaporize or emit nasty smells. Its ionic liquid background means it doesn’t stick to the rules regular organic solvents follow—users find themselves drawn to its stability under harsh lab conditions. Not only does it allow for custom mixing and chemical experimentation, it also acts as a solid electrolyte in research devices. The product often ships in tightly sealed vials to keep moisture and air out, given that many ionic liquids react badly to water or oxygen exposure.
Physical & Chemical Properties
The melting point hovers below 100°C. This trait makes it a liquid at mild temperatures, though it can also exist as solid granules at cooler room conditions. Researchers have spotted a clear, colorless to pale yellow appearance in its molten state. The bromide ion present helps with high conductivity—important when shifting electrons or ions around in a battery or electrochemical cell. Molar mass lands at around 251.13 g/mol. The density, recorded at about 1.4 g/cm³, suits it for density-based separations. Viscosity falls on the higher end, making it resistant to flow and excellent at dissolving polar materials. Moisture uptake remains moderate. Thermal stability stretches up to 250°C in a dry, inert atmosphere, which ensures it survives during long-duration experiments.
Technical Specifications & Labeling
Manufacturers provide [MOE-MIM]Br usually at a purity of 98% or above, with moisture content below 0.2% for technical-grade batches. Labels show batch numbers, net content, molecular formula (C7H13BrN2O), hazard pictograms, and safe-handling warnings. Packaging info appears on the outside—typically glass bottles with Teflon caps. For research labs, a detailed certificate of analysis accompanies each batch, listing any residual starting materials, contaminant levels, and IR/NMR trace spectra.
Preparation Method
Labs mostly prepare [MOE-MIM]Br by N-alkylation of 1-methylimidazole with 2-methoxyethyl bromide, using anhydrous conditions. The starting reactants meet in a polar aprotic solvent like acetonitrile, and the reaction proceeds at moderate temperatures, usually 50–80°C. Vigorous stirring helps complete the process. Product forms as a separate solid or oily layer, washed with cold ethyl acetate or ether to remove impurities. The product then undergoes recrystallization, often from acetonitrile or ethanol. Drying under vacuum removes trace solvents, which is crucial for high-purity ionic liquid work. The yield consistently passes 80% in carefully controlled batches, with low side-product formation.
Chemical Reactions & Modifications
Chemists take advantage of the imidazolium core’s reactivity for functionalization. Introduction of different alkyl or aryl chains on the nitrogen atoms lets researchers tweak solubility and stability. Bromide exchange reactions replace the halide with other counterions, such as tetrafluoroborate or hexafluorophosphate, unlocking fresh properties. Under basic or reducing conditions, the imidazolium ring participates in nucleophilic substitution or even deprotonation, turning the compound into valuable catalysts or precursors. The methoxyethyl tail tolerates mild acid or base hydrolysis but resists oxidation under controlled conditions.
Synonyms & Product Names
On the shelf, the compound may pop up with a few different names. Most commonly, labs record 1-(2-methoxyethyl)-3-methylimidazolium bromide. Chemical suppliers might list it as [MOE-MIM]Br, MOEMIM Br, or sometimes CAS registry numbers like 858742-65-7. Catalogs group it among tailor-made ionic liquids, distinguishing it from its ethyl or butyl cousins. This naming system keeps things organized for researchers jumping between product suppliers or comparing physical and chemical reports.
Safety & Operational Standards
The conversation around ionic liquid safety grows louder as their popularity spreads. Direct skin contact with [MOE-MIM]Br tends to cause mild irritation, and ingestion—while rare—results in stomach upset. Proper PPE, including nitrile gloves and goggles, helps avoid exposure. I remember my own initial experiments: even a small spill left a persistent oily feeling. Labs should run reactions under good ventilation, and spills require quick cleanup with absorbent pads, then a thorough wipe-down. Storage in cool, dry spaces prevents unwanted hydrolysis and decomposition. Waste handling needs careful attention. The bromide component puts the product into a hazardous-waste category in some jurisdictions, so disposal involves high-temperature incineration in approved facilities. Emergency safety data sheets warn of potential but low-level toxicity to aquatic environments if released, reminding users to keep it far from drains.
Application Area
Lab groups use [MOE-MIM]Br in creative ways. Battery researchers mix it with lithium salts to produce high-conductivity electrolytes for experimental energy storage devices. Synthetic chemists dissolve polar or metallic catalysts in it, hoping for better yields and selectivity than with regular solvents. Environmental technologists look into its ability to soak up heavy metals or organic pollutants from water, an approach that keeps showing promise. Some teams try it in extractions, purifying pharmaceuticals or rare-earth metals. Each new application brings in a wave of data, refining our collective understanding of ionic liquids’ strengths and weaknesses. The compound’s reusability after simple washing and its thermal resilience grant it an edge over old-school organic solvents.
Research & Development
Today’s R&D pushes the boundaries on both purity and sustainability. Green chemistry labs look for less toxic routes starting from bio-based imidazoles and greener bromides. Analytical chemists dive into the fine points of how [MOE-MIM]Br dissolves, disperses, or segregates other chemicals. Teams experiment with micro-scale reactors, aiming to scale up processes for real industrial use. One trend involves blending [MOE-MIM]Br with other ionic liquids, tuning mixtures to handle specific challenges—such as the removal of sulfur from fuels or capture of volatile organic compounds. Collaborations between academic groups and manufacturers keep the innovation train moving, as every fresh discovery lands on a journal page or in a patent database.
Toxicity Research
Although ionic liquids in general were once called “green solvents,” more studies hint at environmental challenges. Tests show [MOE-MIM]Br has low acute toxicity to lab animals at typical exposure levels, yet chronic effects remain under study. Aquatic studies demonstrate that even low concentrations disrupt algae and some freshwater invertebrates. The methoxyethyl group resists breakdown under standard conditions, raising flags about long-term persistence. Regulatory bodies ask for more detailed breakdown studies, including how the material behaves in soils and water treatment plants. Having handled dozens of ionic liquids myself, I favor a careful approach: limiting unnecessary use, reusing smaller quantities, and backing all experiments with sound risk assessments. Transparency in safety testing helps build trust, especially as industrial interest grows.
Future Prospects
Looking ahead, the story of [MOE-MIM]Br and similar compounds pushes deeper into sustainable chemistry, clean energy, and high-value manufacturing. Researchers work to shorten synthesis steps, reduce reliance on petrochemicals, and improve the product’s end-of-life profile. As energy-storage technology expands and electrochemical devices become more common, compounds like [MOE-MIM]Br position themselves as indispensable tools for scientists and engineers. Given the rising standards around chemical safety and environmental performance, only those ionic liquids that meet both the productivity demands and the regulatory benchmarks will endure. This pressure fuels both incremental improvements in synthesis and bold innovations in new chemical forms, driving us closer to a future where high-performance materials and green technologies can work hand-in-hand.
Understanding Molecular Details Matters
Curiosity about chemical compounds can be both practical and personal. In a world leaning on advanced materials for everything from clean energy to life-saving drugs, the nuts and bolts of a molecule like 1-Methoxyethyl-3-Methylimidazolium Bromide reach well beyond textbooks. On the surface, it sounds technical, but the details connect directly with real-world innovations. This compound falls into the family of ionic liquids, a category known for unusual properties and big promise in green chemistry circles.
Why Ionic Liquids Stand Out
It’s easy to take solvents for granted until you realize traditional choices often come with environmental baggage. Volatile organic compounds (VOCs) drift off into the air, raising health and safety concerns. Ionic liquids like this one dodge a lot of those problems. They barely evaporate, they don’t catch fire easily, and their unusual structures can be tweaked for different tasks. For me, it rings a bell from a time working in a university lab where solvent fumes meant sore throats and long lectures on safe handling.
The Atom-by-Atom Build
Let’s dig into what makes up 1-Methoxyethyl-3-Methylimidazolium Bromide. The foundation sits with the imidazolium ring — a five-sided carbon and nitrogen structure. In this case, a methyl group tacks onto one of the nitrogen atoms, making it 3-methyl. Next, imagine a 1-methoxyethyl branch attached at the other nitrogen. This feature includes an oxygen atom bound to a two-carbon chain, which finishes with a methoxy group (an oxygen linked to a methyl, CH3O–). The positive charge lands on the imidazolium ring thanks to these substitutions and, to balance things out, bromide (Br–) jumps in as the anion.
Where Fact Meets Application
This structure gives the compound traits that matter in the lab and beyond. The long organic side chains make it less rigid than many salts, which explains why these substances flow like oils at room temperature instead of forming crystals. They’re none too shy about dissolving a range of materials, from dyes to tricky organic chemicals. Academics write about using them as solvents for “difficult” reactions or even electricity carriers in batteries. Over the years, they’ve shown promise breaking down cellulose or cleaning up industrial waste—displacing much more hazardous chemicals.
Reaching for Safer Chemistry
Safe alternatives in the world of solvents deserve attention, mostly because many current routes lean on toxic or flammable chemicals. This compound’s structure cuts down on these risks by trading volatility for stability. Fewer emissions mean less indoor air pollution and less risk for accidental fire, which hits home in both industry and teaching. Bio-based ionic liquids might push this progress even further, trimming the environmental downsides from production to disposal.
Room to Improve
Every innovation runs into roadblocks. Ionic liquids can still be expensive to produce and sometimes prove hard to recycle. Bromide, while less problematic than some, isn’t completely off the environmental radar. Researchers keep working on versions that swap out halides for friendlier ions or use recyclable starting materials. Companies and labs joining forces over the last decade now help steer this chemistry toward fewer hazards and lower costs.
Conclusion? Keep Watching This Space
So, peering at 1-Methoxyethyl-3-Methylimidazolium Bromide unlocks much more than a naming puzzle. Knowing the ins and outs of its structure means learning how safer, more sustainable chemicals get designed. It blends good science with practical improvements—something that anyone breathing easier in a chemistry class or factory can appreciate.
Stepping Into the World of Ionic Liquids
The name 1-Methoxyethyl-3-Methylimidazolium Bromide often lands in the realm of chemistry labs and green technology startups, but behind this tongue-twister is a simple fact: it’s an ionic liquid that keeps chiming for chemists and engineers working with tough separations and energy projects. In my own time at a university lab, standing next to shelves of glass bottles and fume hoods, I spotted this compound tucked in with others under the heading of “alternative solvents.”
The Solvent Powerhouse
People rely on this ionic liquid because it resists evaporation and won’t ignite easily. That stands out compared to classic solvents used for years in the chemical industry. Researchers mix it into extractions or reactions that involve sensitive molecules, because it doesn’t act up and degrade or interfere. This stability means less risk in both research and scaled-up production. Back during a project to clean up organic waste, switching from a flammable solvent to an ionic liquid like this one meant we could run all-day processes with half the drama – no smells, less cleanup, and fewer hazardous waste containers piling up by the door.
Cleaner Chemistry and a Push for Sustainability
As more governments press for less toxic waste from manufacturing — especially in the pharmaceutical field — 1-Methoxyethyl-3-Methylimidazolium Bromide stakes its place as a greener option. For example, synthetic chemists swap in this ionic liquid for processes that strip out target compounds from mixes of organics and metals. Its low vapor pressure also cuts air pollution concerns, a major step up from solvents like ether, which often escape into the atmosphere. Recent reports from EU-funded sustainability initiatives rank these liquids as critical in the move to cleaner process engineering.
Separations and Electrochemistry
Bromide ionic liquids pop up in separation units, helping pull water from alcohol or extract metals from waste streams. This role carries into battery research too. As big names in renewable energy hunt for better electrolytes — stuff that carries charge inside batteries — chemists turn to ionic liquids, trusting their wide “electrochemical windows” (basically, the voltage range they can handle before breaking down) and their tough resistance to temperature swings. Several university labs I’ve visited keep vials of these on hand when testing new battery designs, especially solid-state prototypes.
Down the Pipeline: Catalysis and Material Science
Catalysts thrive on supported environments, so researchers came up with “supported ionic liquid phase” catalysis — using 1-Methoxyethyl-3-Methylimidazolium Bromide as a backing for metal particles. Jobs like hydrogenations, oxidations, or carbon capture suddenly become more efficient and easier to control. Work published by American Chemical Society journals highlights that these liquids don’t just act as bystanders. They stabilize tricky catalysts and often steer reactions toward cleaner, higher-value products. In one project, I saw our lab’s yield double simply by switching over to this kind of ionic liquid.
Challenges on the Table
Cost still bumps up against broad adoption. Compared to long-used industrial solvents, ionic liquids like this aren’t made in massive volumes yet, driving up prices. There’s also a need to pin down their long-term environmental impact. Some might persist in soil or water if spilled, so research keeps digging into how to recover, reuse, or break them down safely. Companies and academic labs are chasing new synthesis routes that cut down on both waste and cost — a good signal that, moving forward, the chemistry world aims for responsible scaling.
What’s Next?
As demands for cleaner energy, safer chemical processes, and resource recovery speed up, expect 1-Methoxyethyl-3-Methylimidazolium Bromide to keep showing up in new places. Its high stability, adaptability, and track record in boosting efficiency give engineers and scientists the tools to take on tomorrow’s toughest separation and synthesis challenges.
Understanding the Chemical
1-Methoxyethyl-3-methylimidazolium bromide isn’t a household name, but it’s showing up more in laboratories and industry research circles. This compound sits in the family of ionic liquids, which serve as unique solvents and catalysts in chemical reactions. Some researchers enjoy ionic liquids for their low volatility and ability to dissolve a wide range of substances, but safety always takes priority.
Potential Hazards Lurking Beneath
Ionic liquids look harmless at first glance, often as clear or faintly colored solutions. That doesn’t mean safe handling can be skipped. Scientists have raised concerns about acute toxicity, skin and eye irritation, and potential environmental harm that comes from mishandling these substances. While articles sometimes tout lower flammability compared to standard organic solvents, that trade-off shouldn't encourage casual methods.
1-Methoxyethyl-3-methylimidazolium bromide has a chemical structure that gives it the ability to disrupt cell membranes and protein structures. I remember a graduate student in my lab who suffered hand irritation after skipping gloves while pouring a similar compound into a beaker. His experience became a lesson to everyone else, underlining the fact that chemical burns and allergic reactions don't discriminate based on the size of the spill.
What Safety Data Tells Us
This compound comes with a Material Safety Data Sheet (MSDS) packed with warnings. Direct skin or eye contact risks redness, pain, or even burns. Inhaling dust or vapors means potential irritation to the nose, throat, and lungs. Laboratory exposures often happen quickly and unpredictably, especially when transferring powders or working with open containers in small spaces.
The Environmental Protection Agency and similar authorities stress avoiding release into drains or soil. Many ionic liquids have persistent effects on aquatic life, persisting long after a spill. In working with any chemical new to the lab, keeping these broader impacts in mind becomes a moral responsibility, not just a regulatory checkbox.
Simple Precautions Save Trouble
Personal stories aside, handling 1-methoxyethyl-3-methylimidazolium bromide comes down to common-sense preparation. Put on chemical splash goggles, nitrile gloves, and a lab coat even for quick tasks. Swap out latex gloves for nitrile ones; the structure of ionic liquids can slip through latex more easily than some realize.
Work inside a chemical fume hood with a working airflow. Fume hoods pull airborne particles and vapors away from breathing space, a vital step since inhalation risks may not create symptoms until hours later. Keep absorbent packs or spill kits within arm’s reach, as these compounds can be slow to mop up and tricky to neutralize once on the floor.
Know your eyewash station location. Keep it clear, unobstructed, and test it daily. If the container arrives with the chemical in powder form, open it gently, tilting away from your face. Scales, spatulas, and containers need careful cleaning every time, because residues can persist and cause harm to the next person who handles them.
Genuine Caution Beats Regret
Experience tells me that most chemical accidents come from letting guards down, even for a second. Each lab incident changes practices for years, forcing better habits. If you're new to 1-methoxyethyl-3-methylimidazolium bromide, treat it with the same care you would for older, well-known risks. Ask for training before solo work, and always review up-to-date safety data. This approach keeps both you and your coworkers protected, and it builds trust in safe research environments where everyone can learn productively.
Understanding What’s at Stake
1-Methoxyethyl-3-Methylimidazolium Bromide belongs to the ionic liquid family, a group I’ve seen grow in popularity in both labs and industry thanks to their unique roles in advanced solvents, electrochemistry, and catalysis. I remember the first time I handled a bottle of this ionic liquid—the label listed storage instructions in tiny print, but the person training me didn’t bother to explain them. A month later, the contents had turned a weird color. That experience taught me that ignoring real-life storage details carries real consequences, like dangerous degradation or loss of that costly chemical.
Heat and Humidity: Enemies of Stability
My old chemistry professor told everyone on the first day of her advanced synthesis class, “Assume every chemical degrades twice as fast in a hot, damp room.” She was right. 1-Methoxyethyl-3-Methylimidazolium Bromide holds up best in a cool, dry spot. Temperatures between 2 and 8°C give the best shot at keeping its structure intact over time. Think of the back of a standard refrigerator—not the freezer and not the shelf over a radiator.
This substance doesn’t play well with moisture. Ionic liquids sometimes pull in water from the air (they’re hygroscopic), which can trigger breakdown reactions and create impure samples. One solution is to seal the bottle tightly after every use. Out on the bench, I’ve seen bottles left open for just fifteen minutes gain measurable weight as they absorbed water. A few labs I've worked in used a glovebox filled with dry nitrogen or argon for storage and transfers, but most don’t have that luxury. Instead, silica gel packs and desiccators are easy fixes. Put the chemical in a small, well-sealed vial, toss in some silica gel, and keep it away from open air as much as possible.
Light and Contaminants: Hidden Hazards
Sunlight is rough on this chemical. Extended exposure sometimes leads it to degrade, losing the qualities researchers paid good money for. Opaque bottles or at least amber glass offer a good shield. I once had a project delayed by weeks because an ionic liquid degraded from sitting under bright bench lights. Since then, I store all sensitive liquids in dark cupboards or covered boxes.
Cross-contamination is another quiet risk. Residues from syringes or spatulas bring metal ions or dust into the mix, accelerating side reactions. Clean, dry tools make a huge difference. I keep a set of glass vials and pipettes just for ionic liquids—saves cleanup time and keeps each compound purer longer.
Beyond the Basics: Tracking and Disposal
Expiration labels matter more than most people think. Investing a little time updating inventory logs or slapping on “opened” stickers saves headaches. In the lab, tracking how long an opened bottle sits can spot stability problems before they sabotage an experiment. If the material smells off or looks cloudy, best to swap it out instead of risking a failed run or safety hazard. Proper disposal follows all local chemical safety protocols—never down the drain, always by the book.
Storing this compound right costs little but pays off in safety and reliability. Taking real steps with temperature, dryness, darkness, and cleanliness preserves not just shelf life but also the odds of successful and safe experiments down the line.
Why Purity Tells the Real Story
Work in any chemistry lab, and purity never feels like a background detail. Impurities mess with results, slow down reactions, and double the troubleshooting. Suppliers for 1-Methoxyethyl-3-Methylimidazolium Bromide typically set the purity bar at or above 98%. Some tout 99% or higher, which often matters for research, advanced synthesis, and quality control in manufacturing. Lower purities might shave costs up front, but headaches soon follow—unexpected peaks showing up during NMR runs, mysterious residues after evaporation, or off-target effects during catalysis tests.
Narrow confidence gaps give chemists room to breathe. You want to trust that if the supplier’s certificate of analysis shows 99%, the next batch will land on your bench with the same numbers. This isn’t just about paperwork. Consistency keeps projects on schedule and means less waste—both reagents and time. Inconsistent batches quickly erode trust. A solid supplier will disclose those trace impurities, and robust labs will double-check with HPLC or NMR, sometimes sending feedback right back so everyone stays honest.
Actual Appearance: What Meets the Eye
Out of every bottle and ampoule I’ve opened, the cleanest batches of 1-Methoxyethyl-3-Methylimidazolium Bromide rarely surprise you. The chemical shows up as a white to off-white powder or sometimes small, crystalline chunks. Some batches lean toward a very faint yellow—usually no cause for concern. If it’s brown, clumpy, or sticky, something has clearly gone sideways: moisture or an oxidized impurity probably crashed the party. Too much yellowing or discoloration hints at incomplete purification or aging, so it never hurts to ask for photos if you’re evaluating new suppliers.
Solid ionic liquids like this one can clump a bit if they sit in humid storage. Still, they should flow easily once broken up or gently dried. Big differences in appearance can signal batch problems, so regular checks save time down the line. Sourcing from a reliable supplier means rarely encountering unexpected textures or colors. Stay alert to shifts in look and feel, since that can point to bigger headaches hiding below the surface.
Quality Control: More Than Just Looks
Firms trading in specialty chemicals face mounting pressure to offer strong quality control. Good labs publish certificate data—purity, moisture, metal ion content, even spectral data. That transparency builds trust, reducing surprises. Certificates backed by HPLC, GC-MS, and NMR numbers allow you to size up the supplier’s standards before a single gram arrives. A supplier that shies away from sharing these details often doesn’t earn repeat business.
Missteps in quality control create ripples lasting months. I’ve seen teams grind to a halt, waiting for answers when a product's off-spec. At its worst, low-quality batches force scientists to repeat work or—harder to catch—let subtle contaminants skew the results. Mistakes set research and industrial scale-ups back, wasting money and sometimes damaging careers.
What Works: Reliable Sourcing and Testing
Besides sticking to reputable suppliers, every lab should keep a few simple checks in their toolkit. Scrutinize every new batch right when it comes in. Run a quick spectrum, double-check purity claims, and log appearance for future reference. Over the years, running these checks became fast habit. While it adds a few hours at the start, it’s much faster than having to redo a week’s worth of experiments or hunting for a contaminant’s source after the fact.
A good supplier listens to feedback, updates their processes, and shares accurate specs up front. Suppliers who don’t keep up with these expectations run the risk of falling behind—or worse, losing customers for good.