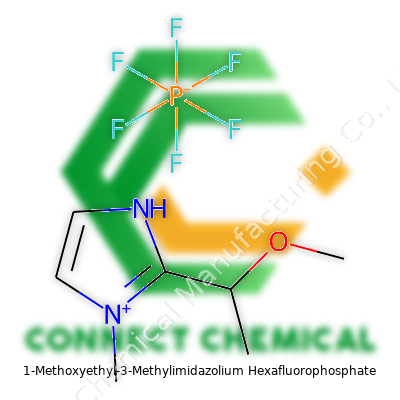
1-Methoxyethyl-3-Methylimidazolium Hexafluorophosphate: A Real-World Look at an Uncommon Ionic Liquid
Historical Development
Chemists searching for eco-friendlier solvents soon found themselves eyeing ionic liquids. Among these, 1-methoxyethyl-3-methylimidazolium hexafluorophosphate (commonly shortened to [MOEMIM][PF6]), started cropping up in technical journals about two decades ago. The early 2000s saw researchers break out from the standard-issue imidazolium salts to experiment with more functionalized side chains, and the methoxyethyl group stuck out as a way to tweak polarity and solubility without veering into volatility. Folks in research labs had their eyes on emissions, reactivity, and safety. The realization that a family of custom solvents could shake up extractive metallurgy, electrochemistry, and organic synthesis provided the drive for developing [MOEMIM][PF6]. Instead of sticking to legacy approaches, labs started adapting syntheses using cleaner, salt-based processes, which let this class of compound gain traction.
Product Overview
1-methoxyethyl-3-methylimidazolium hexafluorophosphate falls under the umbrella of room-temperature ionic liquids. It comes as a colorless or faintly yellowish, oily liquid, less viscous than some of its cousins but much heavier than water. The molecule’s imidazolium core provides stability against decomposition, and the hexafluorophosphate counterion keeps it non-halogenated, both important for industrial application. Manufacturers have been able to scale its production to kilogram levels, though it hasn’t reached the tonnage of more basic ionic liquids due to specialized demand.
Physical & Chemical Properties
This liquid tips the scale at a molecular weight of 282.21 g/mol. Its melting point stays below room temperature, often hovering just above freezing, letting it flow freely in standard lab or plant settings. Its density usually clocks in around 1.3-1.4 g/cm3. Unlike traditional organic solvents, it barely evaporates even when left open—vapor pressure remains close to zero. Its methoxyethyl side chain loosens up the rigid structure seen in earlier, simpler imidazolium liquids, lending it better solvation power for some metal ions and organic molecules, and it tolerates more water before separating. The compound resists burning, and its high ionic conductivity opens it up for electrochemical uses, while the hexafluorophosphate lends remarkable chemical stability, except under strong reducing conditions or at high temperatures where hydrolysis can release hazardous PF5 gas.
Technical Specifications & Labeling
Every bottle of [MOEMIM][PF6] will show the expected purity, which commercial suppliers routinely certify above 98%. Labels typically provide batch number, CAS number (472972-74-2), UN shipping codes, and recommended storage limits, generally sticking close to a cool, dry place away from acids and bases. Certificates of analysis will mention moisture content, halide content, and residual solvents. Researchers and industrial users keep an eye on chloride content, since it hints at incomplete synthesis or possible hydrolytic instability. Technical specifications also call for clear instructions on handling: chemical compatibility charts, electrochemical windows, and shelf life based on container type.
Preparation Method
Chemists can synthesize [MOEMIM][PF6] in a two-step process. First, 1-methoxyethyl bromide or chloride reacts with 1-methylimidazole to form 1-methoxyethyl-3-methylimidazolium halide. This intermediate then undergoes anion exchange with potassium hexafluorophosphate in water or acetonitrile, and the resulting ionic liquid sinks to the bottom, letting the water-soluble potassium halide get filtered off. Drying agents and vacuum steps keep the compound water-free. The real challenge lies in removing all traces of halide and water, since these interfere with high-tech or electrochemical uses. Labs often need repeated washes, drybox storage, and validation by ^1H-NMR and ^19F-NMR, with suppliers providing spectral data to confirm purity.
Chemical Reactions & Modifications
The stability of the imidazolium core resists casual chemical attack, but the ether-linked side chain invites functionalization. Chemists sometimes substitute other alkoxyalkyl or polyether chains to tune viscosity or compatibility with solutes. While the hexafluorophosphate anion generally resists nucleophilic attack, harsh alkaline conditions or intense heating start to break it down. In electrosynthesis, [MOEMIM][PF6] serves as both solvent and electrolyte, allowing smooth electron transfer at the electrode without getting reduced itself. For those looking to tweak electrochemical behavior, swapping the PF6- anion with alternatives like BF4- or Tf2N- yields related liquids with softer or harder acidity, impacting applications downstream.
Synonyms & Product Names
In catalogs and research papers, 1-methoxyethyl-3-methylimidazolium hexafluorophosphate often carries other labels, including [MOEMIM][PF6] and (1-methoxyethyl)-3-methylimidazolium hexafluorophosphate. Some suppliers break it down as 1-(2-methoxyethyl)-3-methylimidazolium hexafluorophosphate, with the 2-methoxyethyl reference pointing out the attachment position. Minor differences in side chain notation sometimes confuse buyers, so researchers double-check NMR data and supplier information before using a new batch.
Safety & Operational Standards
Despite the buzz about ionic liquids being “green solvents,” each one brings particular hazards. [MOEMIM][PF6] demands careful handling. Its toxicity hasn’t been charted as fully as those of chloroform or acetone, but the hexafluorophosphate ion can decompose and release toxic fluoride or PF5 gas if exposed to acids, bases, or high heat. This calls for chemical fume hoods, eye protection, gloves, and grounded storage. Labs use carbon dioxide or dry chemical extinguishers should a fire break out, since water can make things worse if hydrolysis occurs. Disposal by incineration in authorized facilities, never down a sink, sidesteps toxic byproducts. Comprehensive safety data sheets give risk phrases, first-aid advice, and guidelines for storage and spill cleanup. Even as certain regulatory agencies weigh the risks of PF6-, routine health and environmental checks at major users keep these hazards visible.
Application Area
This ionic liquid’s sweet spot lies in specialized domains. Its high polarity and negligible vapor pressure let electroplaters and battery researchers push reaction boundaries that choke off with legacy solvents. In electrodes and supercapacitor research, [MOEMIM][PF6] brings stability and a wide electrochemical window for pushing current without side reactions. Chemists exploring catalysis or extraction use it for dissolving metal complexes, lipophilic compounds, or even waste organic streams, knowing this compound won’t boil off and pollute the air. Some pharma and fine chemical processes benefit from its selectivity in separating active molecules or by sticking to clean synthesis standards. Regulatory scrutiny may slow its use in bulk manufacturing, but research-scale and pilot plant projects continue breaking new ground with applications reliant on its unique solvent properties.
Research & Development
Current research tackles both the advantages and challenges this ionic liquid brings. University labs and industrial R&D groups focus on its use in greener catalysis, hoping to cut reliance on volatile solvents while achieving better yields and selectivity. Teams studying battery technology zero in on its ionic conductivity and electrochemical stability, leveraging its resistance to decomposition. Others, worried about waste streams, run comparative tests against other anions to see if greener alternatives match up in performance. Several groups chase opportunities in waste treatment and rare-earth element recycling, seeking separation processes that outpace traditional liquid-liquid extraction. For every breakthrough on performance, researchers have in mind the regulatory hurdles, cost factors, and safe end-of-life options, reflecting a maturing field that balances promise and caution.
Toxicity Research
Toxicologists haven’t wrapped up the full risk assessment for [MOEMIM][PF6]. Small-scale studies suggest low volatility limits workplace inhalation risks, but the hexafluorophosphate content raises eyebrows. Animal studies on related ionic liquids often show moderate acute toxicity, particularly through oral exposure. Chronic studies remain scarce, and waste management becomes a sticking point because decomposition can unleash fluoride ions, toxic to both humans and aquatic life. Researchers who have trained in green chemistry keep demanding fuller toxicological profiles and push for low-toxicity alternatives whenever possible. Institutions run workplace monitoring and biomonitoring, trying to catch any problems before they snowball into chronic health or sanitation concerns. A lot of ongoing research involves environmental fate and transport, since no one wants ionic liquid accumulation in soil or groundwater.
Future Prospects
Looking forward, demand for premium functional solvents hinges on both technical gains and regulatory clarity. Companies investing in advanced batteries and electrorefining watch closely to see if end-of-life recycling and fluorine management can keep pace. The hunt continues for ionic liquids combining performance with safer anions and non-toxic side chains. Advances in separation science and green chemistry push industry and academia to engineer new molecules modeled on [MOEMIM][PF6], hoping to combine water stability, safety, and high performance. Folks in the know keep a cautious optimism about scaling up production, as long as industry partners tackle waste safely and support next-generation process design built on lessons learned from the past two decades of ionic liquid research.
Understanding This Unique Ionic Liquid
Anybody working with advanced materials or chemistry labs will have run into ionic liquids. 1-Methoxyethyl-3-Methylimidazolium Hexafluorophosphate doesn't come up in conversation outside the world of research or specialty manufacturing, but it earns attention for a reason. The chemical structure combines properties chemists crave—stability, low volatility, and a knack for dissolving substances other solvents can't touch. People in my field spot its greenish credentials right away.
Electrochemistry and Batteries
The push for stronger and safer batteries keeps getting louder. What most don’t see is how traditional electrolytes—a core part of every battery—struggle when pushed to higher voltages. 1-Methoxyethyl-3-Methylimidazolium Hexafluorophosphate helps solve this. This ionic liquid doesn't evaporate easily, cuts down fire risk, and keeps batteries running even at tougher temperatures. Lithium-ion research papers show it supports better conductivity than the standard organic blends. Researchers test it for supercapacitors too, since it resists breakdown and won't dry out like older chemicals. Motors running on renewable power depend on exactly these gains, so this liquid has earned its spot on every serious battery developer’s list.
Solvent for Difficult Reactions
I’ve watched synthesis teams struggle getting reactions to finish, especially those that fail with conventional solvents. 1-Methoxyethyl-3-Methylimidazolium Hexafluorophosphate takes substances normally impossible to dissolve and brings them into solution. Pharmaceutical chemists use it for stubborn reactions and for making complex molecules you’ll see in tomorrow’s medications. Since this solvent resists water, it opens doors for moisture-sensitive experiments—everyone who’s lost a run to an unexpected water leak remembers the pain.
Catalysis and Chemical Separations
Industries ranging from refinery operations to specialty plastics like finding ways to recycle metals or clean up chemical streams. Using this ionic liquid as a support or co-catalyst, engineers can strip out rare metals like platinum from waste mixtures or recover gold by tweaking the fluid’s properties. My industry friends who work in recycling point out that conventional solvents often pull in too many impurities, but this kind of compound can target the valuable material much more selectively. Environmental impact shrinks and companies keep expensive raw materials moving back into production, not buried in landfills.
Green Promise and Precautions
One of the big selling points for ionic liquids always comes down to safety and the environment. Volatile organic compounds turn up everywhere—from cleaning products to fuel cells—and they come with hazards. 1-Methoxyethyl-3-Methylimidazolium Hexafluorophosphate stays stable at normal conditions, and doesn’t vaporize easily, which means less air pollution in workplaces. Its low flammability appeals to labs and manufacturing plants. Chemists push to replace some of the old, toxic solvents with ones like this—though everybody agrees we have to stay aware of how these new materials behave over long periods, including how they break down.
A Few Challenges Worth Solving
Cost comes up every time someone presents a new process using specialty ionic liquids. Scaling from the bench to a full factory floor feels daunting, since the raw materials take skill and energy to make. I’ve seen promising methods using recovered components and better recycling systems, which look set to cut supply chain problems. Building green chemistry skills throughout the sector remains key. Students and professionals alike need stronger training programs, so that breakthroughs in labs don’t get lost before real-world adoption.
Stability Comes Down to Good Habits
Every chemist remembers the first time they lost an entire sample to careless storage. That harsh lesson teaches you to pay attention to what a compound actually needs, not just guesswork. Chemicals have quirks and weaknesses — some break down with air, others hate moisture, and a few even freak out around sunlight. Understanding these oddities is what makes the difference between getting the results you want and wasting time on ruined batches.
The most fragile chemicals often react with light or oxygen. A classic example: silver nitrate tends to turn dark because it’s light-sensitive. Store it in amber bottles and keep it away from direct exposure to the lab’s fluorescent lights. Sodium metal, famous for its drama with water, must stay submerged in mineral oil. Someone may shrug and ask why it matters. Ask yourself how much a ruined experiment costs in reorders and wasted effort. Relying on experience and common-sense protocols isn’t busywork. It saves resources in the long run.
The Details Matter: Temperature Makes or Breaks Stability
One common mistake is assuming that refrigeration always helps. Cooling slows down decomposition for many compounds — but not all. Freezing can damage some solutions by causing them to precipitate, separate, or become less effective. Take organic peroxides; they can explode if crystallized in the cold. For many biological reagents, keep them just chilled, not frozen. Manufacturers' literature and chemical safety data sheets aren’t window-dressing. Over my years in the lab, pulling out the technical sheet became second nature before stashing anything.
Iodine sublimes at room temperature and loners like lithium aluminum hydride degrade with air and water. Desiccators work well for both, as long as the silica gel inside stays fresh. Sealed containers with gas-tight lids, combined with proper labeling, help avoid embarrassing surprises. Trying to skip steps leads to those “learning moments” that everyone secretly regrets.
Handling and Storage: Not a One-Size-Fits-All Game
Big mistake: storing incompatible chemicals together or letting acids and organics share a cabinet. Storing concentrated ammonia near bleach risks a dangerous reaction if they spill. Facts like this aren’t scare tactics. They remind us that organized storage prevents more than just contamination — it keeps people safe. Use separate, labeled cabinets and make sure new team members know the system. No one wants to triage an emergency because someone put peroxides near flammable solvents.
Even sealed containers don’t last forever. Caps fail, threads wear out, and chemicals can slowly leach into the plastic. Scheduling inventory checks once a month saved my old lab from losing a batch of sensitive aldehydes that developed a nasty odor from air exposure. A quick sniff or visual inspection before each use catches many problems early.
Solutions That Actually Work
Get organized. Don’t let “it’s always been done this way” stand in for smart protocols. Keep guides and datasheets handy; don’t let pride get in the way of double-checking. Invest in proper storage — dry cabinets for sensitive reagents, fireproof lockers for flammables, and separate bins for acids and bases. Never underestimate the value of a simple desiccator or a working refrigerator. Add a logbook to track shelf life and lot numbers. It takes discipline, not fancy equipment or endless rules, to keep your chemicals safe and ready to use.
Years of experience show that careful handling isn’t just for compliance — it protects projects, budgets, and people. Stay alert to changes, talk with experienced colleagues, and never hesitate to upgrade your system if you spot weaknesses. Good storage is a habit, not a chore.
Why Hazard Reviews Matter
Most people don’t think twice before grabbing a cleaner from the shelf or handling materials in the shop. I’ve noticed that labels often tell part of the story, but not all of it. Some products give off fumes or cause a rash after just a few minutes of use, even when you think you’re being careful. Others seem harmless until you read the fine print—then you understand why there’s a skull and crossbones or an exclamation mark stamped on the side.
Industry watchdogs and agencies like the Occupational Safety and Health Administration, and the Environmental Protection Agency, regularly flag certain chemicals and products as hazardous. Ammonia-based cleaners, chlorine bleaches, and paint thinners can react with each other or irritate the skin and lungs. Product recalls happen every year because of hazards nobody imagined when the item hit the market. I once worked near a shop where improper mixing of cleaners led to an evacuation and folks rushed outdoors coughing and rubbing their eyes. It taught the whole crew never to take clear liquids at face value.
Real Dangers People Ignore
Everyone has that friend who shrugs off warnings. “Been using this for years, never had an issue.” Until one day, the cap slips off a bottle, or gloves tear, or a kid gets curious. Hazardous products live in nearly all homes: pesticides under the sink, gasoline in the garage, aerosol cans in the closet. If a chemical can burn skin, irritate lungs, or poison a pet, it deserves respect and preparation.
Mixing bleach and ammonia can produce toxic gases. Solvents in paint strippers can soak through skin or release vapors that make you dizzy. Even simple things like essential oils, if spilled on the skin or swallowed, send people to the emergency room every year. Poison centers and ER doctors agree: most of their injury calls come down to skipped instructions or ignored warnings.
Smart Safety Precautions
Rushing through a task rarely saves time if it leads to injury. Always check the product’s label, search for its safety data sheet online, and ask for help if any detail doesn’t make sense. Use gloves rated for the type of chemical—latex or nitrile for many solutions, but always check compatibility. Eyes matter too; cheap glasses work better than squinting and hoping for the best. Open a window or run a fan if the bottle or can says “use in a well-ventilated space.”
Store dangerous products in original containers. Never transfer bleach or gasoline into old soda bottles—children mistake them for something they can drink. Keep products high up or inside cabinets with childproof locks if little hands are around. Never mix household cleaners outside of what the label suggests. Wash hands and arms thoroughly after handling, and throw away dirty rags or sponges that may catch fire if left bundled up.
Better Safety Habits Start with Small Changes
Taking common-sense steps keeps families, workers, and pets safe. Local hardware stores and online resources make it easier than ever to find clear safety information. I’ve seen the results of carelessness, but also the payoff from reading instructions and gearing up right. Good habits spread quickly when people share their experiences and keep safety top of mind every time a product comes out of the cabinet or warehouse.
Looking Beyond the Datasheet
1-Methoxyethyl-3-Methylimidazolium Hexafluorophosphate might sound like something only a handful of people handle, but if you’ve ever worked in a chemistry lab, you’ve probably seen a bottle of it. Most suppliers ship this ionic liquid at a purity level of 98% or higher. That number on the label means more than checking a box. Every percent of impurity can throw off research, affect solvent performance, or throw contaminants into the reaction mix. I’ve seen research projects stall because a single batch came in just under spec.
Purity in the Real World
Many chemical providers—Sigma-Aldrich, TCI, Alfa Aesar—market this compound at a minimum of 98% purity. Chemists tend to push for 99% or even better when reactions get sensitive, but the step up in price usually kicks in above that threshold. For someone synthesizing materials where tiny impurities shift conductivity or reactivity, even that last 0.5% can mean contamination. Routine analytical work or screening might do fine with 98%, but anything heading to published results, or towards a device, leans on the higher end.
Why Not Just Buy Ultra-Pure?
Higher purity always costs more. That extra cost comes from painstaking purification—think repeated recrystallizations, lots of solvent use, and seriously careful drying processes. If you’re buying large amounts, that markup grows quickly. Infrastructure for storing and handling ultra-pure chemicals adds another layer. Many labs just can’t justify the spend unless absolutely necessary.
It’s not just about budget. In practice, chasing 100% creates diminishing returns. A product labeled 98% often tests higher in reality, but every supplier runs their own quality checks. Ask for the Certificate of Analysis and you’ll find trace amounts of water, starting materials, and maybe byproducts—usually measured in parts per million. For some applications, like as an electrolyte in batteries or as a solvent in catalysis, even those slip into the “important to monitor” category.
Staying Safe with the Right Grade
Lab mishaps sometimes start with purity confusion. Most researchers can spot obvious impurities, but the trickier ones—like moisture—may not show up until your yields start to dip or your NMR spectrum throws out unexpected peaks. Investing in Karl Fischer titration or advanced analytical tools makes a difference. I learned early that detergents and tap water can add their own surprises to a supposedly pure batch, especially with ionic liquids that love to grab water right out of the air.
Solutions: Testing, Sourcing, and Bench Habits
Chemists who need the purest compounds go beyond just trusting supplier grades. Running in-house checks, keeping samples in a desiccator, and building relationships with reputable suppliers add real value. Some researchers even request custom purification or micro-scale synthesis when high purity is non-negotiable.
Communicating closely with vendors helps iron out doubts. Asking for regular analytical updates, storing chemicals properly, and training new lab members on best practices reduce surprises down the line. Striving for perfect purity creates its own challenges, but knowing exactly what’s going into each experiment builds reliability and real confidence in each result.
The Risks of Cutting Corners
Many folks see ionic liquids as miracle chemicals—green solvents, barely flammable, low vapor pressure, and low volatility. That kind of reputation invites a dangerous trap: tossing safety to the side once their job is finished. I’ve worked around enough labs and plants to see what shortcuts can cost. Even though these liquids don’t give off much vapor, plenty of them break down under heat or light, building up toxic byproducts. Some leach trace metals, others resist water treatment altogether. These issues don’t fit the old guesses around solvent safety.
What Science Says
Research tells a stubborn story. Some ionic liquids, especially those with fluorinated or phosphonium groups, linger in soil and water, hitting aquatic organisms hard. Data from the American Chemical Society highlights imidazolium-based liquids as acutely toxic to fish and daphnia at low concentrations. Others trigger mutagenic changes in exposed bacteria and yeast. Environmental agencies from Europe and the US have tagged certain ionic liquids for tight restrictions—especially once they’re spent and possibly even nastier than they started. It’s just not worth treating these leftovers like old acetone or ethanol.
Practical Steps in Disposal
In my early lab days, we bagged anything with sketchy labels and stuffed it in waste drums, hoping the contractors would sort things out. That’s the sort of luck you find before a landfill catches fire. Now the smarter approach starts in the laboratory notebook—write down exactly which ionic liquid you’re using, log what gets mixed in. Catalogue everything, right down to the rinses.
After that, check the local environmental laws before even thinking about the drain. In the US, most spent ionic liquids tick enough boxes on the Resource Conservation and Recovery Act to classify as hazardous. This means they should head to certified chemical waste disposal, where specialized incinerators run hot enough to break tricky bonds and their exhaust systems trap the worst gases. Industrial partners often need full documentation and original safety data sheets. Missing paperwork means extra fees or entire drums bounced back to your dock.
Cleaner Alternatives and Good Habits
Curiosity has driven a bigger trend toward using more biodegradable ionic liquids. Several research teams have started to push choline-based compounds, which break down more cleanly and don’t bioaccumulate. They offer solutions for industries hungry for less risky processes without the threat of persistent waste. If your process can swap for a greener solvent, folks in the maintenance crew and the local watershed both benefit.
Any disposable gloves, glassware, or tools contaminated with used ionic liquids should land in a dedicated chemical waste container. No shortcuts, because a trace left behind in a sink or trash pile can build up over months. I’ve pulled clogged drain traps from lab sinks that reeked of past mistakes—nobody wants the cleanup job when regulations catch up, not to mention the environmental risks.
Responsibility Doesn’t End With Use
Once an ionic liquid finishes its run, the job’s only halfway done. Following standard protocols and keeping clear records saves money, hassle, and maybe a few gray hairs. Beyond regulatory needs, it’s simple common sense—these chemicals support greener technology only if their leftovers don’t cause more harm in the long run.