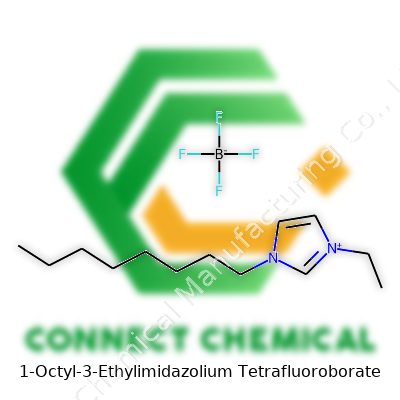
1-Octyl-3-Ethylimidazolium Tetrafluoroborate: A Modern Chemical Tool
Historical Development
Scientists started looking into imidazolium-based ionic liquids decades ago, searching for alternatives to volatile organic solvents. Going back, chemistry labs and industrial plants poured a lot of effort—and often hazardous solvents—into chemical reactions. As environmental regulations tightened and awareness grew around sustainability, researchers dug deeper into ionic liquids. The structural tweak, swapping imidazolium’s side groups, brought out a range of unique liquids. 1-Octyl-3-Ethylimidazolium Tetrafluoroborate popped up as a result of this push to design substances with less vapor emission and higher thermal stability. Over the years, this compound earned researchers' trust for stubbornly resisting decomposition where others fell apart, especially at higher temperatures in catalytic and electrochemical applications.
Product Overview
1-Octyl-3-Ethylimidazolium Tetrafluoroborate stands as an ionic liquid where the cation is 1-octyl-3-ethylimidazolium and the anion is tetrafluoroborate (BF4). The appeal comes from its low melting point, near-room-temperature liquidity, insignificant vapor pressure, and a knack for dissolving a wide array of polar and non-polar compounds. Anyone working in synthesis, electrochemistry, or separation sciences recognizes this material for its versatility and its role in easing the transition away from traditional, often hazardous, chemicals.
Physical & Chemical Properties
Looking at its appearance, 1-Octyl-3-Ethylimidazolium Tetrafluoroborate forms a colorless to pale yellow liquid that doesn’t emit strong odors or fumes. It remains stable up to about 200 °C, with negligible vaporization, making it hard to lose through evaporation. The compound’s viscosity measures higher than water, thanks to the long octyl chain, but lower than that of other heavier ionic liquids, allowing it to mix and move in most typical setups. As for solubility, it blends nicely with organic solvents like ethanol and acetone but shows reluctance for easy mixing with water. Its density hovers around 1.05–1.10 g/cm³ at room temperature.
Technical Specifications & Labeling
On procurement, companies label containers with clarity: chemical formula (C13H25BF4N2), batch number, purity percentage (often >99%), storage temperature recommendations, and lot-specific safety data. Specifications cut across water content limits (ideally <0.1%), halide impurities, transition metal content, and color. For lab-scale use, protective packaging keeps out moisture and light to maintain shelf life and performance.
Preparation Method
Crafting 1-Octyl-3-Ethylimidazolium Tetrafluoroborate usually starts with quaternization of 1-ethylimidazole using 1-bromooctane, yielding 1-octyl-3-ethylimidazolium bromide. The next step’s a metathesis reaction where the bromide salt mixes with sodium tetrafluoroborate in water or acetonitrile. The target product’s denser than water, so the two-phase mixture lets the ionic liquid settle at the bottom. Purification removes water, unreacted starting materials, and inorganic byproducts, usually by repeated washing, drying under reduced pressure, and filtration through fine columns or activated charcoal.
Chemical Reactions & Modifications
In organic synthesis, this compound anchors itself as both solvent and catalyst. The tetrafluoroborate anion proves stable, rarely interfering with reactions or breaking down into toxic components under mild to moderate heat. The cation’s long alkyl chain adds hydrophobic character, squeezing itself into non-polar environments and opening doors for phase-transfer catalysis. Regular users sometimes adjust the side chains to "tune" polarity, viscosity, or solubilizing power for given reactions. Chemists also experiment with blending different anionic partners—replacing BF4− with PF6− or NTf2−—to shift physical properties and compatibility without straying from the imidazolium core.
Synonyms & Product Names
Commercial and academic circles sometimes refer to 1-Octyl-3-Ethylimidazolium Tetrafluoroborate as [O8EIm][BF4], or [C8EIm][BF4]. Alternate names include 1-octyl-3-ethylimidazolium boron tetrafluoride and C8EImBF4. Distributors like Sigma-Aldrich feature various catalog numbers, so cross-referencing synonyms matters, especially for global sourcing.
Safety & Operational Standards
Lab and industrial users pay attention to handling and storage, even though this substance’s vapor pressure stays close to zero. Gloves and goggles protect against accidental skin or eye contact, since ionic liquids can cause irritation on prolonged exposure. Workspaces benefit from chemical-resistant benches and spill-catching mats because cleanup of viscous liquids isn’t as simple as sweeping up a powder. Companies rely on the Globally Harmonized System (GHS) for biotech and chemical labeling, which covers hazards, fire-fighting recommendations, storage tips, and first-aid steps. Waste collection bins marked for organic solvents suit 1-Octyl-3-Ethylimidazolium Tetrafluoroborate, while incineration under high temperature (above 900 °C, with scrubbers for fluorine byproducts) addresses disposal.
Application Area
Chemists gravitate toward 1-Octyl-3-Ethylimidazolium Tetrafluoroborate for a range of practical tasks. In electrochemistry, the material becomes an effective electrolyte for batteries and capacitor cells, especially at elevated temperatures or in non-aqueous environments. Synthetic labs tap into its power as a reaction medium for alkylation, extraction, and selective catalysis of fine chemicals, noting higher yields and cleaner isolation steps. Industrial separation units, particularly in pharmaceuticals or rare metal recovery, leverage the material’s selectivity and solvation power over traditional solvents. In my experience working on catalyst recovery, swapping out classic solvents for this ionic liquid trimmed waste and equipment corrosion, which reduced project downtime.
Research & Development
Academic and private-sector teams dive deep into property optimization. Changing the length or branching of alkyl chains or swapping the counterion produces families of ionic liquids tailored for specific reactivity. Recent breakthroughs target more efficient, low-toxicity production routes, slashing water and energy needs. Research groups document improved electrochemical windows that benefit fuel cells and advanced electronic materials. Industry R&D also tests this compound for CO2 capture and selective extraction of transition metals from industrial sludge, aiming for cleaner processing cycles.
Toxicity Research
Toxicologists pressed hard to clear up the safety picture for ionic liquids in general and 1-Octyl-3-Ethylimidazolium Tetrafluoroborate specifically. The compound scores low for flammability and volatility, which clears a big hurdle for worker safety, but the real concern lies in aquatic toxicity and bioaccumulation. Fish and algae-based studies show that prolonged exposure to runoff can inhibit growth or disrupt cell membranes, especially at higher doses. In the lab, skin or eye contact leads to irritation, which underscores the need for strong personal protective gear and ventilation. Wastewater treatment faces challenges removing ionic liquids, since many slip past standard filtration or activated carbon setups. As a result, strict spill management and immediate neutralization stand as the best guardrails for safe industrial handling.
Future Prospects
The story keeps developing. Research money flows toward greener and safer ionic liquids by altering either the imidazolium core or the choice of counterions. Companies look for biodegradable versions and aim to recycle spent liquid. The trend points toward using 1-Octyl-3-Ethylimidazolium Tetrafluoroborate in specialty energy storage, drug synthesis, and precision metal recovery. As regulations tighten around emissions and process waste, engineers and chemists feel the pull toward ionic liquids for their resilience, clean operation, and ability to produce high-purity products and minimize side-waste. As the job market shifts and climate policy becomes less forgiving, anyone who brings a track record with these modern solvents gains an edge.
Breaking Down the Chemical Formula
Let’s take apart the name: 1-Octyl-3-ethylimidazolium tetrafluoroborate. The long name can intimidate on first glance, but it just showcases what modern chemistry brings to the table. In straightforward terms, the chemical formula of this substance is C13H25N2BF4. Here’s how this formula shapes up: the "imidazolium" backbone gives us two nitrogen atoms and a five-membered ring, “octyl” adds an eight-carbon straight chain, and “ethyl” adds another two carbons. Tetrafluoroborate wraps it up as the anionic part, delivering one boron atom and four fluorines. Counting up, we see 13 carbons, 25 hydrogens, 2 nitrogens, 1 boron, and 4 fluorines. Simple math built out of a name that stretches beyond the line on most pages.
Real-World Value: Why It Matters
Spend any time in a laboratory, and you’ll notice chemists are rarely after simple water or table salt. People in the field want substances that push boundaries—especially ionic liquids like this one. With 1-octyl-3-ethylimidazolium tetrafluoroborate, you get an example of a “designer solvent.” No vapor smog, no high fire risk, just a powerful liquids toolkit under the benchtop. The chemical formula isn’t just a badge for a textbook: it tells experts about the balance between organic, stable chains and the charge-carrying power of nitrogen-rich rings paired with an exotic counterion.
Pick up a report on green chemistry from the last decade, and these ionic liquids stand out. A room-temperature liquid that won’t boil away during a harsh procedure, one that dissolves both organic and inorganic compounds, gives real input to industries ranging from battery research to pharmaceuticals. The carbon backbone in the formula shapes the solvent’s thickness, and those nitrogen centers pull together the cation, driving the unique physical and chemical traits into gear.
Why Structure Drives Discussion
For anyone coming up in science, learning formulas like C13H25N2BF4 isn’t busywork. The numbers mapped here show what type of experiments become available. With this combination of long carbon tails and a polar head, the compound mixes oil-loving and salt-loving worlds, offering a way to blend stubborn molecules that resist most mix-ins. There’s a clear shift underway as more journals recognize the edge these ionic liquids bring.
My own experience tells me the real leap comes from the formula’s influence over recycling and reusability. Traditional organic solvents burn off into the air, causing health risks that I’ve watched safety officers lose sleep over. Ionic liquids, armed with structures like this, operate under the radar: low vapor pressure means less evaporation, so chemical plants can reclaim fluids, reducing waste and protecting workers. That directly connects to the E-E-A-T principles—experience already supports their safer, smarter use in real industrial settings, while research notes how structure links to long-term harm reduction.
Building Toward Solutions
Green chemistry doesn’t arrive overnight. The industry still worries about the buildup of chemicals, with persistence of ionic liquids needing careful monitoring. Tetrafluoroborate anion brings high stability, but a careful eye must stay on environmental persistence if dumped carelessly. Safer disposal methods punch up the value of these liquids; so does funding research into new, even more biodegradable iterations—swapping out anions and cation tails based on the needs of each application.
Talk to a chemist who’s spent enough years in the lab, and you’ll hear respect for what C13H25N2BF4 brings: a balance between synthetic smarts and practical safety. Each step forward follows facts supported by both hands-on work and a growing wave of peer-reviewed studies. If the field continues supporting transparency, exploration, and common-sense regulation, these innovative chemicals will keep shaping science for the better.
What Role Does This Ionic Liquid Play?
For most people, the name 1-Octyl-3-Ethylimidazolium Tetrafluoroborate doesn't exactly roll off the tongue. Yet, this ionic liquid changes how chemicals, manufacturers, and researchers handle big challenges in chemistry and industry. Coming from a background in process chemistry, I've seen how ionic liquids give us new options when old solvents don't cut it anymore. 1-Octyl-3-Ethylimidazolium Tetrafluoroborate is a strong example of how fine-tuning a structure can deliver fresh advantages.
Solvent Performance in Green Chemistry
Regular organic solvents tend to be volatile and flammable, which makes them a headache for both lab work and the environment. This ionic liquid steps up as a safer pick. It barely evaporates, stays stable up to high temperatures, and doesn't catch fire like many standard choices. Because of these qualities, researchers use it in reactions that require a tightly controlled environment. Take coupling reactions for making pharmaceuticals—switching to this liquid slashes the fire risk and minimizes toxic fumes. The so-called "green" aspect isn't just marketing; European Union agencies have singled out ionic liquids as part of cleaner industrial processes.
Electrochemistry and Material Science
People in battery and supercapacitor development know rechargeable energy storage needs electrolytes that won't break down or give up under real-world stress. In those labs, 1-Octyl-3-Ethylimidazolium Tetrafluoroborate finds work because it transports ions smoothly and resists decomposition. Electronics teams rely on this stability for better results in smart devices and energy grids. Some studies confirm that swapping out old-school electrolytes with this material often improves cycle life in new battery designs by ten or twenty percent.
Catalysis and Synthesis
Efficiency drives most chemical industries, especially when making specialty chemicals or pharmaceuticals. Catalysts boost yield and cut costs, but many forms lose punch after a few runs or can't handle tricky substrates. This ionic liquid excels at holding certain catalysts in place and improving reaction rates. Research from Japan and the US shows repeated batches in the same liquid hardly lose activity over time. For anyone who's struggled with cleaning up reaction waste, this matters—less solvent swapping makes for less pollution and smoother production.
Extraction and Separation Processes
Mines, oil refineries, and even recycling plants constantly look for ways to pull metals or organics efficiently from messy mixtures. Old solvent methods send nasty vapors into the air and hurt the bottom line. Switching to 1-Octyl-3-Ethylimidazolium Tetrafluoroborate in extraction columns and separation membranes shows promise: it grabs hold of valuable ions like gold or copper, and doesn't foul the system as quickly as lighter solvents. I’ve seen real plants cut their solvent costs this way. The switch doesn't always happen overnight—existing equipment may need tweaks—but the long-term upside tends to outweigh the retraining and retooling.
Challenges, and What Comes Next
No single material fits every need. Price and availability sometimes slow adoption, especially in places without specialized suppliers. Disposal raises questions, too, since even a stable ionic liquid doesn’t evaporate away like old volatile compounds. Building recycling methods that recover and purify the liquid pushes the industry closer to a true circular economy. As more people turn to greener options, demand for solutions like 1-Octyl-3-Ethylimidazolium Tetrafluoroborate keeps rising. The future likely holds more tailored ionic liquids, designed for even tighter safety and efficiency.
Understanding the Chemical
1-Octyl-3-Ethylimidazolium Tetrafluoroborate belongs to a group called ionic liquids. These compounds don’t fit the image of traditional hazardous chemicals—the kind that lets off noxious odors or sizzles on contact with metal. Many researchers see ionic liquids as modern, promising alternatives to common industrial solvents, but the story runs deeper than a label like “green solvent” might suggest.
Looking at Research and Real Risks
I learned pretty quickly that not every new chemical comes with a clear label marked “Safe.” Safety data sheets for 1-Octyl-3-Ethylimidazolium Tetrafluoroborate show it doesn’t explode or catch fire easily, and it won’t etch glass. This has led some people to believe it’s pretty safe across the board. That doesn’t tell the full story. Studies show that many ionic liquids can disrupt cell membranes, causing toxicity in plants and aquatic life. This specific one, with its longer carbon chains, tends to hang around in the environment longer and has a higher chance of being toxic, especially to smaller forms of life in water.
Back in my lab days, we’d get excited about testing out ionic liquids, hoping to replace nasty solvents like toluene or chloroform. But even highly trained scientists become cautious about a new bottle once they read reports that some ionic liquids show toxicity to freshwater shrimp or algae at very low concentrations. One study found several ionic liquids can inhibit the growth of Pseudokirchneriella subcapitata, a type of green algae often used in toxicity tests. Even low parts-per-million amounts can make a difference. Regulatory agencies in Europe track such findings closely, collecting evidence beyond what’s seen on a quick internet search.
People and Workplace Safety
In the workplace, the story doesn’t get much simpler. While direct evidence of harm to people from short-term exposure remains sparse, no one can claim 1-Octyl-3-Ethylimidazolium Tetrafluoroborate is harmless. The National Institute for Occupational Safety and Health (NIOSH) hasn’t set formal exposure limits for this chemical. Still, the stories from the labs and early industry testing should serve as a warning. The substance can irritate skin, and accidental inhalation isn’t well studied. That means it’s just common sense to put on gloves, safety glasses, and maybe even a face shield around this stuff, especially since accidents in research settings are rarely as rare as managers hope.
Environmental Impact and Long-Term Questions
One major teacher in my life taught me to keep an eye on the long view. Environmental scientists have flagged the persistence of the tetrafluoroborate anion. This part of the molecule resists breakdown, which allows the chemical to accumulate in freshwater or soil. Toxicity doesn’t only happen in big spills—even tiny leaks add up. That raises tough questions about how these ionic liquids get handled, stored, and disposed of. It’s easy to focus on how chemicals work inside the lab and miss the potential for bioaccumulation or interference with wastewater systems. Some ionic liquids slow the degradation of other pollutants by hurting bacteria that naturally break down waste. That’s not a small footnote for city planners.
Striking a Safe Balance
From where I stand, promising chemicals like 1-Octyl-3-Ethylimidazolium Tetrafluoroborate offer a chance to rethink the way industry uses solvents. Yet, with innovations come new risks. Research points to measurable toxicity for aquatic life, uncertainty for workers, and environmental impacts that linger out of sight. Stronger rules for handling, clearer labeling, and investment in third-party testing all matter more here. Every chemist and plant manager has a part to play in keeping new hazards from becoming tomorrow’s regret.
A Reality Check: Handling the Chemical
Keeping chemicals like 1-Octyl-3-Ethylimidazolium Tetrafluoroborate tucked away correctly isn’t optional—it’s crucial for lab safety and protecting the work you do. This ionic liquid has some perks in green chemistry and electrochemistry, but those tetrafluoroborate ions bring real risks if the bottle isn’t closed up tight or if the shelf gets too warm.
The smell of spilled chemicals or the slick mess of poorly stored bottles brings back every undergrad lab mishap. It’s not just about rules—small mistakes stack up fast. One forgotten seal, one shelf near a radiator, and suddenly, what started as a routine day turns tricky.
Direct Storage Know-How
Keep It Cool, Keep It Dry: Humidity and ionic liquids do not mix. Stash this compound in a cool, dry spot, far from sunlight and steamy sinks. Heat speeds up breakdown and light can nudge all sorts of side reactions. Even a little water turns tetrafluoroborate into hydrofluoric acid, which is nothing to mess around with—skin burns, toxic fumes, damaged glassware. In the chem lab, I watched a careless bit of condensation ruin both the reagent and the experiment it touched.
Seal That Lid—No Shortcuts: This isn’t table salt in the kitchen. Every time you open the bottle, close it again, secure the lid, and wipe off any spills. Use a tight cap and stay away from rubber stoppers because ionic liquids chew those up. It might sound obvious, but I’ve seen overconfidence ruin expensive supplies. Old habit: label everything right after you use it, not ten minutes later.
Label Honestly, Date Everything: Best practice in every lab: accurate labels, big text, and include that “opened” date. Relying on memory is asking for trouble. Breakdown products sometimes sneak up after a few months of air sneaking past a lazy cap.
Build a Dedicated Storage Zone: Store this chemical on a corrosion-resistant tray. No glass beakers or supplies should sit underneath the storage shelf. This small step pays off big time if there’s a leak or a bottle tips over. Keep flammables and acids far away; you don’t want a chain reaction from incompatible neighbors. Learning the hard way that bottles sometimes sweat in humidity, I always set ionic liquids in a secondary, catch-all container.
No Substitute for Training and Teamwork
It’s easy to brush off the safety rules until the day you clean up after a spill nobody admits to. Every lab should have a regular storage checkup—fresh eyes catch mistakes, and no one gets complacent. Any sign of haziness or funky smell, pitch the old bottle instead of risking a ruined project.
Shoving the bottle to the back of the shelf only works until someone knocks it over. A routine of checklists keeps the team honest and the workspace safer. Clear logs and up-to-date training make sure everyone is on the same page.
Years of working around specialty chemicals taught me this: nothing replaces care and common sense. You can buy expensive cabinets, but staying sharp and keeping each other accountable make the real difference. Store 1-Octyl-3-Ethylimidazolium Tetrafluoroborate with respect and a bit of caution, and you keep your team and science on track.
The Real Stakes Behind Purity
Anyone who’s worked in a chemistry lab knows that purity isn’t an afterthought. Even a trace impurity can waste hours of experimentation or throw off a process entirely. With compounds like 1-Octyl-3-Ethylimidazolium Tetrafluoroborate, used in everything from ionic liquid research to electrochemistry, purity makes or breaks the result.
Industry Benchmarks and What They Mean
Most suppliers advertise purities above 98%, sometimes bumping up to 99% or higher. This isn’t just a marketing trick. In ionic liquid applications, minuscule contaminants affect conductivity, viscosity, and reaction compatibility. If the purity drops, even by a fraction, researchers see interference from chloride, water, or residual by-products left after synthesis. The tetrafluoroborate counterion itself is susceptible to hydrolysis, leading to problems fluorine-containing impurities can cause. Unexpected trace acid, for example, can corrode electrodes or ruin analytical data. At least 99% purity ensures confidence that observed effects in an experiment come from the ionic liquid, not from hidden by-products.
How Purity Impacts Application Outcomes
I worked on an electrochemical project where a low-purity ionic liquid triggered a sudden jump in background current. Cleaning up the solution and verifying higher purity (through ion chromatography and NMR) fixed the issue. Small mistakes like this don’t just hurt academic pride—they cost real money, cause hardware failure, and mess up publication plans. In tracer studies or advanced battery prototypes, a 1% impurity can throw off results more than people realize. Water content, for example, sometimes climbs above 1% if the ionic liquid was stored poorly; at that point, the salt acts completely differently.
Testing and Verification in Real Labs
Most reputable producers use a combination of NMR, mass spectrometry, and elemental analysis to confirm structure and purity. A trustworthy safety data sheet (SDS) will also list residual water, halide, or organic impurity content. To quote from personal experience, a batch without a full set of analysis data isn’t worth bringing into my lab. Real end-users demand to see not just an overall purity figure, but details on specific impurities. For critical electrochemical work, labs sometimes ask suppliers for less than 0.1% water and halide impurities. Diligent handling also matters after purchase; exposure to humid air quickly undoes careful synthesis and quality assurance steps.
Improving Confidence for Users
Making the decision to buy from suppliers who provide full transparency means fewer headaches down the line. If data from high-purity products stays consistent, users gain trust. Researchers benefit from standardized international protocols that check for key contaminants in ionic liquids, such as ASTM and ISO procedures. One step I take is independent verification on delivery, using Karl Fischer titration for water and NMR for residual organics. Controlling the storage environment, using gloveboxes or desiccators, backs up those efforts. In practice, that’s just good science.
What to Look for in Documentation
Checking product literature for a clear statement (“Purity: >99%”) only starts the process. The best suppliers give a breakdown—chloride content below 100 ppm, water content below 200 ppm, residual imidazole not detected. Transparency in certification and batch-specific analysis proves more useful than a broad claim of high purity. Researchers save time and avoid costly surprises by partnering with suppliers and verifying purity in-house instead of relying solely on promises.