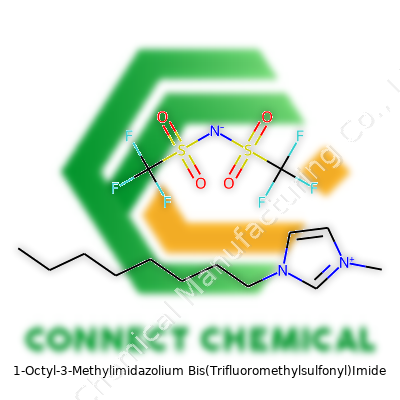
1-Octyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide: Unlocking Ionic Liquid Potential
Historical Development
Decades ago, the hunt for safer and more sustainable solvents led chemists down some unlikely paths. Few could have predicted that ionic liquids—liquids made entirely from ions—would shake up traditional thinking about solvents the way they have. Among them, 1-Octyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (OMIM TFSI) stood out. Research through the 1990s turned up the limitations of conventional organic solvents: flammability, volatility, and toxicity that lingered long after use. Trial after trial made it clear that simply swapping one hazardous solvent for another wouldn’t cut it. That’s where OMIM TFSI earned its stripes: as someone who’s spent years in the lab fighting with nasty solvents, the arrival of such stable, tunable liquids felt long overdue. The ionic liquid boom changed the game, especially as strict regulations forced industries to rethink solvent risks and environmental impact.
Product Overview
OMIM TFSI brings to the table low volatility and chemical stability even under tough conditions. Its structure—a cation with an octyl group attached to a methylimidazolium ring, married to a heavily fluorinated TFSI anion—helps it dodge hazards like flammability and corrosiveness. Industries often demand extreme versatility from materials like this. Unlike many specialty chemicals, OMIM TFSI doesn’t just get used up; it recycles and repurposes easily thanks to robust thermal and chemical stabilities. Over the years, global manufacturers and research labs built trust in this chemistry, not just for convenience but because making good chemistry safer is a responsibility that sits with every user.
Physical & Chemical Properties
Anyone trying to handle tricky reactions or sensitive extractions knows the value of a solvent that won’t disappear at the first sign of heat or air. OMIM TFSI shows a melting point that usually sits well below room temperature, letting it perform in the liquid state under most conditions that matter. Viscosity tends to stay manageable, neither too thick nor annoyingly runny, making pouring and pumping straightforward even at scale. Hydrophobicity stands out as well. Unlike older imidazolium salts, this one handles water poorly—and that’s a strength, since many separations count on solvents not picking up unwanted water. Chemically, OMIM TFSI shrugs off oxidation, reduction, and most acids and bases. That’s a breath of fresh air for those who spent years trying to salvage sensitive products from solvent decomposition or acid breakdown.
Technical Specifications & Labeling
Technical data for OMIM TFSI provides the backbone for regulatory compliance and safe application. Purity typically runs north of 98%, which matters for catalysis and electrochemical experiments. Density lands around 1.3 g/cm³ at 25°C, and thermal decomposition rarely starts before 350°C. Conductivity and viscosity aren’t just trivia points; they determine how well this chemical fits roles from battery electrolytes to extraction solvents. Proper labeling includes hazard identifications, especially the presence of fluorinated components and warnings about persistent environmental effects. Careful attention to GHS pictograms, transport restrictions, and Material Safety Data Sheets (MSDS) separates responsible use from accidental hazards.
Preparation Method
Synthesis of OMIM TFSI sticks to routes that avoid introducing water or sensitive side products. Alkylation of 1-methylimidazole with 1-chlorooctane lays the foundation, producing 1-octyl-3-methylimidazolium chloride as an intermediate. Metathesis with lithium bis(trifluoromethylsulfonyl)imide then yields the target ionic liquid. Filtration and repeated extraction with dry organic solvents strip away contaminants. Many research groups emphasize purification by activated charcoal treatment and vacuum drying to drive purity higher. Every time someone runs a reaction, the quality of the starting materials makes or breaks the result—and that’s been true long before ionic liquids hit the scene.
Chemical Reactions & Modifications
OMIM TFSI rarely stands still in a beaker. It partners with a range of catalytic species, especially when transition metals or organocatalysts are in play. Its stability, a point of pride, stems from strong bonds in both cation and anion. Direct modifications typically focus on the imidazolium cation—altering the alkyl chain or inserting functional groups to tweak solubility or compatibility with polymers. Functionalization around the TFSI anion remains rare since changing this part risks losing benefits like hydrophobicity and thermal endurance. As a solvent, OMIM TFSI fosters reactions from Diels-Alder to Michael additions, providing a gentle alternative to classic solvents that often brought too much reactivity. Chemists repeatedly see how swapping in OMIM TFSI can shift equilibria, speed up rates, or boost selectivity.
Synonyms & Product Names
The chemical catalog may list OMIM TFSI under a string of names, making accurate sourcing important for traceability. Synonyms like 1-octyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide and [OMIM][TFSI] creep into product sheets, as do abbreviations like C8mim-TFSI. CAS numbers bridge confusion in international trade, as product names can bounce across languages and regulatory environments. In labs worldwide, researchers keep records matching precise product codes to batches—an approach that’s saved more than a few experiments from costly errors due to mix-ups with similar-sounding chemicals.
Safety & Operational Standards
Safe handling never takes a back seat with OMIM TFSI. Its remarkable chemical resistance doesn’t free users from the obligation to treat all ionic liquids with care. Direct contact warrants good gloves, since skin permeability of some ionic liquids remains an open question. Inhalation risks are lower than the classic volatile organics, yet ventilated hoods and eye protection stay essential. Spills wipe up without the fire risk tied to ethers or hydrocarbons, but disposal still counts; the persistence of the TFSI anion means drains and general waste are out. Many countries now expect detailed environmental assessments for new chemicals, and OMIM TFSI gets scrutinized for eco-toxicity and long-term breakdown products. Storage in tightly sealed, dry containers ensures shelf life, sidestepping the slow hydrolysis that eats away at lesser quality ionic liquids.
Application Area
OMIM TFSI carved out a niche in areas that value clean, stable solutions. Electrochemistry, especially advancements in battery electrolytes and supercapacitors, depends on its wide electrochemical window and negligible vapor pressure. Labs engaged in organic synthesis appreciate its ability to host catalytic cycles that struggled in traditional solvents. Extraction of metals from ores—particularly rare earths—harnesses its selectivity and resilience against decomposition. Pharmaceutical research puts OMIM TFSI to work as both a solvent and a stabilizer for drug formulations that degrade in water or alcohol. Industrial lubricants and heat transfer fluids lean on its high temperature endurance and low volatility, extending equipment life and reducing emergencies in manufacturing. After working with ordinary solvents in these industries, switching to an ionic liquid as reliable as OMIM TFSI reduces stress around both safety and process stability.
Research & Development
The research community has driven OMIM TFSI into new territory over the past decade. At conferences, researchers swap application notes showing advances in dye-sensitized solar cells, fuel cells, and even protein crystallization that rides on the unique environment OMIM TFSI delivers. Green chemistry isn’t just a slogan—it has become a competitive advantage in both academia and industry as stricter environmental rules demand cleaner, safer practices. Few areas have seen as much innovation as the coupling of OMIM TFSI with nanomaterials, where it can stabilize particles or tune surface chemistry in ways water and hydrocarbons never could. Each application brings new lessons about solubility, selectivity, and recycling that feed back into process optimization and next-generation design.
Toxicity Research
Research continues to dig into OMIM TFSI’s toxicity, a topic that matters just as much as performance. Acute oral or dermal toxicity tests show generally low immediate hazards, especially compared to legacy solvents like toluene or chloroform. Chronic effects, on the other hand, require careful attention. Degradation products of the TFSI anion and the imidazolium ring can build up in aquatic environments, so long-term ecological studies have become standard before new industrial uses ramp up. Personal experience in the lab echoes the academic literature: even compounds that seem benign in the short run can turn troublesome after years of unchecked waste. For OMIM TFSI, companies with robust handling and disposal practices avoid these pitfalls by treating every batch as a potential long-term environmental factor.
Future Prospects
R&D keeps churning out new uses for OMIM TFSI, driven by the demand for materials that deliver both performance and safety. As battery and capacitor research accelerates—chasing greener, longer-lived storage solutions—electrolytes like OMIM TFSI look poised for even greater roles. Drug synthesis, extraction, and formulation continue to lean on its chemical resilience. Regulatory bodies look to set firmer standards for life-cycle assessment and disposal, requiring producers to innovate cleaner synthesis and reclamation. Companies have started investing in recycling infrastructure for ionic liquids, preventing environmental build-up and shifting OMIM TFSI into closed, controlled loops. As someone who’s seen science adopt and discard solvents over decades, this feels different—more responsive to societal needs, more transparent about safety, and better equipped to tackle the complex chemistry facing future generations.
Understanding a Modern Chemical Tool
I spent a few years working in a chemistry lab that always seemed to be on the hunt for better solvents. It became clear to me that the materials you pick shape the results you get—sometimes in ways you never saw coming. Take 1-Octyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide, a name that would trip up anyone who hasn’t stared at enough bottles on the shelf. The lab folks call it [OMIM][Tf2N], and it sits in a growing family called ionic liquids.
What Makes It Stand Out in the Crowd of Chemicals
Most standard lab solvents—think ethanol, acetone, old-school stuff—do only so much. Something different happens with an ionic liquid like OMIM Tf2N: it brings almost zero vapor pressure, it doesn’t flash off, and it can often replace solvents that give off fumes or catch fire. I saw researchers switch over when running reactions demanding steady temperatures or environments that don’t need water creeping in everywhere. Unlike the classic “one bottle for every job” mindset, this chemical fits roles in several fields because its tuneable structure can give it new tricks.
Industrial and Laboratory Roles
A friend working in battery research kept raving about this ionic liquid. He explained how OMIM Tf2N works as an effective electrolyte, especially when testing out lithium-ion cells. The stability and non-flammability he pointed out have real impact in making storage that doesn’t burst into flames. The chemical’s wide electrochemical window makes it easier to keep batteries performing well, even after heavy use.
It doesn’t stop at batteries. I crossed paths with a group synthesizing organic compounds who appreciated that OMIM Tf2N makes extraction and separation of chemicals on a lab scale faster and sometimes cleaner. In industrial processes, people have tested it out for removing mercury from gas streams and cleaning up polluted water. In these scenarios, the idea that the liquid doesn’t evaporate or mix easily with water lets it scoop up contaminants and get recycled in the process line, saving money and cutting hazardous waste.
What You Won’t See on a Safety Data Sheet
A couple of projects used OMIM Tf2N because it’s considered “greener” than volatile organic solvents. It’s non-volatile, so lab techs breathe easier and there’s less to vent at the end of the day. Still, nothing in chemistry comes without baggage. The stuff doesn’t biodegrade easily. If a spill happens or a batch is discarded wrong, that waste will stick with us. The challenge becomes balancing safety in day-to-day handling with long-term impacts. Anyone using this has to track how much gets recycled versus dumped and keep an eye on where it travels after use—a task the industry needs to keep improving.
Moving Forward with Purpose and Awareness
Better chemicals can push research and industrial safety ahead, but only as fast as people commit to following up with good practices. Many regulations have evolved to keep up, but fewer walk the walk if it gets expensive or slows down production. As more companies explore ionic liquids, it matters to tie every innovation back to environmental checks and a clear plan for handling waste. I’ve learned that every change in solvent or material makes a dent in the bigger system, and the people who measure and plan for these changes end up leading the field.
Understanding Ionic Liquids
Ionic liquids have earned a lot of attention over the past decade. Unlike regular salts, they remain liquid at surprisingly low temperatures. I remember my first time looking into them, I expected a thick, hard-to-handle material, but these substances flow more like oil than rock salt. Most liquids in this category come with low volatility, which means you’re not breathing in their fumes as you might with many organic solvents. They have a knack for being thermally stable, so even when heating them in the lab, there’s little worry about sudden breakdown or nasty surprises.
Main Properties
The biggest feature that keeps coming up is their low vapor pressure. Laboratory spaces benefit from this property. Anyone who’s ever struggled with headaches from regular solvent fumes can appreciate a product that stays put in its container. Thermal stability also goes a long way. There were days in graduate research when samples needed careful heating—organic solvents often smelled up the entire floor, but ionic liquids stuck around without boiling off.
Other common features include electrical conductivity and the ability to dissolve a broad variety of compounds. These liquids conduct electricity because the charged particles freely move around. Mixing polar and nonpolar chemicals becomes easier with an ionic liquid. This dissolving strength suits teams handling specialty extractions or new battery technology. The customizability is significant. Small tweaks to the compound allow scientists to adjust for performance.
Key Benefits
Ionic liquids don’t just reduce headaches from fumes—they can boost safety, cut waste, and even improve final product quality. The environmental side stands out. Many classic solvents are toxic or flammable, and handling them means keeping fire extinguishers nearby. My old research group once switched to ionic liquids for a tricky extraction purely out of safety concerns. The workbench stayed neater, and nobody worried about breathing dangerous vapors.
Their large temperature window translates into new research tools. They work from below zero to above two hundred degrees Celsius, giving flexibility for all kinds of new chemical reactions. Solubility for high-value materials, such as rare earths or pharmaceuticals, opens doors for companies looking to extract value from low-yield resources without clogging pipes or reacting dangerously. I’ve seen electroplating researchers swap out traditional baths for ionic liquids, finding cleaner end results thanks to the ionic liquid’s gentle touch.
Practical Solutions from Research and Industry
Many teams put them to use in green chemistry, replacing volatile organics in synthesis. The result is often an easier cleanup, especially in pharmaceutical labs. Energy storage is another winner—Ionic liquids found their way into batteries, where their stability helps keep fires and leaks to a minimum. Their ability to dissolve waste or tough grime got the attention of recycling companies, who use them for difficult metal recovery.
Researchers still look for ways to cut costs, and bulk production scales have helped make ionic liquids more accessible. Waste management, safer labs, and new types of materials come with regular use. The next time a hazardous solvent needs replacing or lab air gets stuffy from fumes, ionic liquids offer a practical option. Their usefulness isn’t just rooted in performance but in keeping people safer and supporting a cleaner world.
What Are We Really Dealing With?
Faced with a chemical like 1-Octyl-3-Methylimidazolium Bis(Trifluoromethylsulfonyl)Imide, often called OMIM-TFSI, I can’t help but think back to my time in a research lab. One lesson always stood out—never get too comfortable because “safe” means different things in a chemistry lab than in most places. Even clear, colorless, and low-odor liquids have a way of biting back.
The Allure of Ionic Liquids—and Their Hazards
OMIM-TFSI comes from a class called ionic liquids. These liquids get praised for their low volatility, which can make them less likely to evaporate into the air you breathe. I appreciated not having a room full of fumes after hours of electrochemistry work. Yet this doesn’t mean a free pass on safety routines. Low volatility only solves one problem. Touch is another story. This compound stings in small cuts and can linger on your skin, getting absorbed without much warning. Safety data sheets warn about possible eye damage, skin irritation, and respiratory risk from dust or heated vapors. Always respect the material, even if it seems “green” or “benign.”
What Happens If You Don’t Take Precautions?
The data behind OMIM-TFSI’s effects on health is still building. Early studies show that long exposure can lead to trouble, particularly for those with preexisting sensitivities. Some research points to possible damage to aquatic organisms if it gets washed down the drain. As someone who has dealt with chemical waste rules, I know how easily bad habits sneak in—rinsing beakers out of convenience adds up. Often, these compounds resist easy breakdown, lingering in the water supply.
Personal Safety Comes Down to More Than Gloves
Too many folks treat a pair of nitrile gloves and some splash goggles as all the protection they’ll ever need. With ionic liquids, you need to check your gear. Not every glove works. Some weaker gloves let these chemicals soak through. I always double-checked with the safety officer before using something unfamiliar. Long sleeves, a sturdy apron, and a tightly fitted mask protect you from accidental spills or splashes—especially during transfers or cleaning up. Eye contact hurts, and it doesn’t wash away easily. My rule: behave as if every contact could be a problem, because one slip can show you why safety matters.
Waste Disposal Takes Priority
Responsible handling goes beyond keeping yourself safe. Disposal presents an ongoing challenge. Pouring OMIM-TFSI down the sink is a shortcut that risks more than a lecture from the environmental officer. State regulations classify many of these ionic liquids as difficult to break down. Most labs collect them in tightly sealed containers, calling for specialist disposal services. It adds paperwork and costs, but the alternative can harm drinking water and future research.
Steps Toward Safer Practice
Lab safety means consistent vigilance. Good ventilation matters. A hood keeps stray vapors away from your breathing space. Always label containers clearly. Training for both new and experienced staff builds the right habits, especially in fast-paced labs where it’s easy to slip up. Sharing these warnings and keeping MSDS sheets nearby can save a lot of heartache. Every time I see someone take safety shortcuts, I remember the chemical burns suffered by a colleague. Quick action and honest conversations matter more than any theory about “green” chemistry.
Don’t Be Fooled by Reputation
Even substances whispered about as “eco-friendly” bring risks. My experience shows that safe handling has to start with respect—for the compound, for the environment, and for everyone in the room. OMIM-TFSI carries promise for batteries, sensors, and solvents, but only if safety stays at the core. That extra care today prevents big regrets down the line.
Real-World Lessons from the Pantry and the Lab
In the kitchen, it’s obvious. Store bread on the counter and it grows stale or moldy. Tuck it in the freezer and it lasts for months. The same logic applies to products found in every industry, whether it’s something you eat, slather on your skin, or use to clean your car. Storage guidance matters because proper information saves money, preserves quality, and can even keep people safe.
Why Storage Conditions Change Everything
Products respond to their surroundings. Light, heat, moisture, and air each play a part in how long a product stays good. High humidity turns powdery cake mix into stubborn clumps and can make electronics fritz out early. Bright sun breaks down vitamins and drinks. Too much heat wilts medications and curdles dairy before the printed date.
Relying on the right container is just as important as the air around it. I keep my coffee beans in a dark jar with a tight lid, not just because it looks nice, but because oxygen and sunlight turn coffee “off” far too fast. In work settings, this can mean the difference between a safe chemical and something unpredictable. Clear rules around lids, seals, and proper containers help avoid messes, accidents, and wasted cash.
Reading Dates: The Fine Print Isn’t Just Legalese
Every product has its window of high quality. “Best by,” “use by,” and “expires on” all look the same until you get sick from expired yogurt or diluted medicine. Food waste in the U.S. reaches tens of billions of pounds each year, much of it tossed because people misread those dates. Clarity matters. If storage instructions say “refrigerate after opening,” ignoring the warning can make something go foul fast, no matter the date on the package.
Cutting corners can cost plenty. Consider medicines. A study by the World Health Organization shows that poor storage—say, stashing insulin outside a fridge in a hot place—can make it lose punch long before the date. The key is understanding that shelf life isn’t static. It shifts with heat, light, moisture, and even the amount of air left in the bottle after opening.
What Gets in the Way of Doing Things Right?
Often, people store things based on what’s convenient instead of what works. In the bathroom, folks like to stash all their pills above the sink, but steamy showers can damage those products. Turning to pantries, not everyone deciphers the manufacturer’s tiny icons (the ones that show a little fridge, a sun with a line through it, or a jar with a lid popped open).
It’s easy to blame the consumer, but part of the issue falls on manufacturers. Tiny font, unclear symbols, and generic advice do little good. Companies that switch to plain language, bigger images, and vivid colors on labels help families and workers avoid costly mistakes.
Practical Steps for Better Storage and a Longer Shelf Life
Knowledge beats confusion. Smart companies hand out cards or slap QR codes right on their product with simple storage rules. Employees get refresher training, not dry lectures but hands-on demos. In homes, big, legible instructions build good habits. Leftover soup in my fridge sports masking tape with the “make” date so nobody risks a gamble with old leftovers.
No one likes waste. Keeping things in the right spot and following clear directions buys time, saves money, and keeps people healthy. It’s less about memorizing chemistry and more about noticing what works for your situation and reading the label with care. These little habits pack a punch in the long run.
Looking Past Jargon: What Does This Compound Really Offer?
1-Octyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide (often called [OMIM][NTf2]) might sound like a mouthful, but in the laboratory, folks talk more about what it can do than how to pronounce it. I’ve spent a few years watching researchers work with ionic liquids, and this one has carved out a special place as a solvent in electrochemical setups. It doesn’t evaporate at room temperature like water or traditional organic solvents, and that means fewer worries about changing concentrations during long experiments. Its chemical stability holds up under harsh conditions—high voltages, aggressive chemical environments—where glassware usually gets nervous.
The Meaning of Low Volatility in Real Setups
Walk into an electrochemistry lab, and there’s always talk about fumes or accidental spills. You don’t get much of that with [OMIM][NTf2], since it barely evaporates at all. Low volatility means safer working conditions, and no constant topping up or sealing. Beyond health, this saves time and budget on safety equipment like ventilation and fume hoods.
Ions, Charge, and Moving Parts
People sometimes forget the unsung value of a wide electrochemical window. [OMIM][NTf2] lets you work at higher voltages compared to water- or acetonitrile-based solutions. This opens doors for new redox couples, challenging batteries, and tricky electrode materials. In my graduate days, we used it to test metal electrodes that would have fallen apart in acidic or basic solutions. It let us chase curious electrochemical events without seeing glassware shatter or electrodes dissolve.
Stability Is Not Just an Academic Point
If you’ve ever tried running an all-night cyclic voltammetry experiment, you know how annoying it feels to wake up to a dry cell or a failed run. [OMIM][NTf2] resists decomposition, both under air and high potentials, so setups keep working hour after hour. Researchers in battery labs tell me that some lithium-ion and next-generation batteries see lower side reactions because the electrolyte just shrugs off water or oxygen traces. This higher quality keeps results consistent and repeatable, day after day.
Not All That Glitters: Viscosity and Cost Barriers
Of course, [OMIM][NTf2] doesn’t solve every problem. It’s thicker than water—sometimes much thicker. This slows down ions and makes current flow less efficient, especially at low temperatures. I’ve sat through many meetings where people argued about how to thin down the liquid or heat up their system to keep reactions moving.
And it isn’t cheap. Every time an undergraduate spilled a vial, my advisor’s face would drop. Budget-minded labs sometimes use smaller cells, batch processes, or recycle the ionic liquid to save money. That’s a far cry from the “just add more” approach you get with cheaper solvents.
Where Do We Go from Here?
For researchers, smart workarounds help tame viscosity, from stirring and heating to adding cosolvents in small doses. Companies have started blending different ionic liquids to hit a sweet spot between performance and price. Safety training now features ionic liquids as a regular part of the curriculum, not just a curiosity.
[OMIM][NTf2] finds a home where traditional solvents fall short. Its toughness and broad electrochemical window let folks see reactions and store charge in ways other liquids can’t. Cost and viscosity don’t have simple fixes, but as more companies scale up production, prices inch down and blends get better. For now, it walks a fine line between specialty and standard, offering options for people eager to push electrochemistry a bit further.