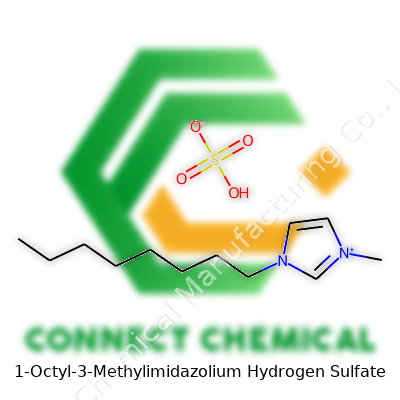
1-Octyl-3-Methylimidazolium Hydrogen Sulfate: An In-Depth Commentary
Historical Development
Chemists began exploring ionic liquids like 1-Octyl-3-Methylimidazolium Hydrogen Sulfate as they searched for alternatives to volatile organic solvents in the late twentieth century. Original experiments with imidazolium salts date back to the 1970s. Green chemistry’s rise gave this field new life in the 1990s, as the industry recognized both the environmental hazards and the process inefficiency tied to conventional solvents. Researchers found 1-Octyl-3-Methylimidazolium Hydrogen Sulfate stood out for its thermal stability, broad solvating abilities, and customizable chemical makeup. Its lower vapor pressure offered tangible safety improvements in industrial settings, which pushed interest beyond academic circles into real-world applications.
Product Overview
This compound, often found in the form of a colorless to pale yellow liquid, belongs to the family of imidazolium-based ionic liquids. Strong ionic character, coupled with an organic tail, gives it a unique profile among liquid salts. Chemists and engineers often choose this specific salt for its performance in acid-catalyzed reactions and separations, as it holds protons tightly while offering wide solubility with both organic and inorganic substances. On my visits to chemical labs, I have seen 1-Octyl-3-Methylimidazolium Hydrogen Sulfate being measured out, its higher viscosity compared to water a noticeable trait. In practice, the handling and storage of this liquid remain straightforward when kept in airtight containers and away from extreme moisture.
Physical & Chemical Properties
With a melting point that sits well below room temperature, this ionic liquid remains stable across a useful temperature range. 1-Octyl-3-Methylimidazolium Hydrogen Sulfate resists evaporation, even in heated environments, which lowers air-borne contamination and the risk for chemical workers. The extended octyl group on the imidazolium cation increases hydrophobicity, boosting its ability to dissolve nonpolar organics. Viscosity can change with temperature, so technicians tune process parameters for optimal flow and mixing. Its hydrogen sulfate anion provides strong acidity—an advantage for catalysis and extraction, especially when paired with the compound’s excellent ionic conductivity. Stability stands out, with the liquid resisting significant decomposition unless heated beyond 200°C or exposed repeatedly to strong bases.
Technical Specifications & Labeling
The highest-grade 1-Octyl-3-Methylimidazolium Hydrogen Sulfate arrives with a purity above 99%, and moisture content often kept well below 0.1%. Labels list all specifications clearly: molecular formula (C12H24N2O4S), batch number, date of manufacture, and storage advice. Reputable suppliers include hazard identification codes, signal words aligned with GHS standards, and recommended handling protocols right on the bottle. These details give buyers a sense of security, knowing they can trace and audit their materials back to their source if quality issues or safety incidents ever arise.
Preparation Method
Producers usually create this ionic liquid by reacting 1-methylimidazole with 1-chlorooctane under nitrogen, generating 1-Octyl-3-Methylimidazolium chloride, then perform anion exchange by reacting the product with excess sulfuric acid. This stepwise process generates hydrogen sulfate as the counterion. Skilled chemists favor this route because it scales efficiently from gram to multi-kilogram batches without excessive by-product formation. Proper purification follows, using either repeated washing with organic solvents or ion-exchange resins, with careful drying under vacuum to remove traces of water. Production facilities, in my experience, focus heavily on minimizing contamination by using air- and moisture-free setups, recognizing the product’s sensitivity to both conditions.
Chemical Reactions & Modifications
1-Octyl-3-Methylimidazolium Hydrogen Sulfate serves as a medium or catalyst in diverse synthesis reactions, especially where both acidity and ionic conductivity help boost reaction rates. Researchers have published protocols using this liquid for esterifications, alkylations, and certain metal-catalyzed couplings, taking advantage of its ability to stabilize charged intermediates. Modification often means swapping out the octyl group for other alkyl chains or exchanging the hydrogen sulfate anion for alternative functional anions to fine-tune solubility or reactivity profiles. These tweaks expand the compound’s reach, proving valuable for colleagues in pharmaceutical synthesis as well as researchers working on greener industrial processes.
Synonyms & Product Names
Lab scientists call this substance by several names, including [OMIM][HSO4], 1-methyl-3-octylimidazolium hydrogen sulfate, or even its abbreviation “C8MIM HSO4.” Some catalogs simply list it under its CAS number. This variety in nomenclature sometimes causes confusion, especially for buyers looking for the correct product. Suppliers keep up with demand for synonyms by updating webpages and catalogs, making ingredient tracking easier for researchers and compliance teams. Academic papers tend to settle on the long-form IUPAC name or the well-accepted short forms, smoothing interdisciplinary collaboration.
Safety & Operational Standards
Best practices for handling 1-Octyl-3-Methylimidazolium Hydrogen Sulfate rely on using chemical-resistant gloves, laboratory coats, and safety glasses. Though much safer than volatile organic solvents, this liquid can irritate eyes, skin, and mucous membranes, especially after long exposure. Proper training in chemical hygiene and the use of local exhaust ventilation limit chronic risks in industrial or academic settings. Disposal runs through solvent recovery programs or licensed waste handlers, as ionic liquids resist biodegradation. Facilities display clear signage and emergency response information in storage and usage areas. Process engineers monitor both temperature and mixing rates during large-scale reactions, as the product’s viscosity affects transfer and stirring when making or using this ionic liquid in reactors.
Application Area
Chemists across industries value 1-Octyl-3-Methylimidazolium Hydrogen Sulfate for its adaptability. In synthetic chemistry, it functions as both catalyst and solvent, offering mild, recyclable conditions for acid-catalyzed reactions. Extractive metallurgy professionals use it to recover metals from ores or e-waste, leveraging its selective solubility and ionic strength. Researchers working in biomass conversion mix it into cellulose or lignin processing, where traditional solvents struggle, cutting down reaction times and boosting product yields. Electrochemists in battery and sensor work test this ionic liquid as an electrolyte for its thermal stability and low vapor pressure, especially where leak risks or temperature swings loom large. In my own lab, the difference in yield and workup simplicity can’t be overstated, especially when compared to traditional mineral acids or volatile organic solvents.
Research & Development
Investments in R&D pour into this area, as both private industry and university labs chase lower carbon footprints, improved safety, and tighter process controls. Work centers on making the synthesis greener by switching to renewable feedstocks, curbing waste, and streamlining purification. Teams experiment with modified imidazolium structures to see how tweaking the alkyl group or anion changes solubility, toxicity, or environmental impact. New findings in catalysis, extraction, and energy materials come out regularly, with researchers looking for both incremental gains and breakthrough applications. I have followed these trends for years, noting the gradual shift from academic novelty to serious industrial contender as companies begin integrating these ionic liquids into scaled production lines.
Toxicity Research
Studies of ionic liquids, including 1-Octyl-3-Methylimidazolium Hydrogen Sulfate, highlight low flammability and lower acute toxicity compared with many organic solvents, yet concerns persist for chronic ecological effects. Aquatic toxicity studies point to moderate persistence and bioaccumulation, as complex cations and anions break down slowly in the environment. Health researchers focus on occupational exposure risks, especially during handling of large volumes, since the irritant nature of the compound rises at higher concentrations. Regulatory agencies follow developments closely, calling for more long-term studies on chronic health effects, biodegradation pathways, and potential alternatives for less-toxic ionic liquid formulations. Personal observation tells me that routine risk assessments and the use of engineering controls in modern labs cut accident potential markedly.
Future Prospects
The market for 1-Octyl-3-Methylimidazolium Hydrogen Sulfate looks set to expand as sustainable chemistry advances. Companies invest in closed-loop processing and recycling to contain both costs and environmental impact. Engineers push to design more efficient reactors tailored for high-viscosity ionic liquids, trimming energy needs and improving product yield. Advances in toxicology provide clearer guidelines, underpinning safer workplace standards and smarter waste handling. The need for greener industrial processes drives innovation, so variants of this ionic liquid with improved properties start to reach pilot plants and, eventually, full production. As each new use builds trust in the technology, adoption spreads, moving away from just specialty chemicals and into wider industrial and research contexts.
Why Chemists Turn to This Ionic Liquid
Anyone who’s spent time in a lab knows how tricky it can get trying to separate or dissolve stubborn materials. Running a reaction sometimes feels like coaxing a mule up a steep hill. Researchers who look for easier, safer methods have turned more often to a group of chemicals called ionic liquids. Among them, 1-octyl-3-methylimidazolium hydrogen sulfate has racked up attention for a few reasons.
Pushing Past Water: A More Versatile Solvent
Solvents keep chemistry running. Water works for most things but doesn’t cover every base, especially for organic reactions. I’ve watched coworkers grimace through failed experiments using water or common organics like acetone. This ionic liquid changes the landscape, handling polar and nonpolar compounds, and keeping things stable at higher temperatures. Its chemical structure gives it the upper hand in dissolving a broad range of substances, and it doesn’t catch fire like diethyl ether or spit out toxic fumes like dichloromethane.
Cleaner Solutions for Industry and Research
Big chemical plants often dump out hazardous liquid waste. The push for greener chemistry makes people look twice at what they pour down the drain. 1-octyl-3-methylimidazolium hydrogen sulfate doesn’t evaporate or pollute air the way old-school solvents do. It plays a big part in reducing workplace hazards and cleaning up the environmental footprint. A 2022 review in Green Chemistry described major reductions in toxic emissions when companies swapped older solvents with ionic liquids.
Catalyst and Reaction Partner
Besides its role as a solvent, it steps up as a catalyst. Certain chemical reactions—like acid-catalyzed esterification or transesterification—run smoother and faster, and the ionic liquid can be recycled a few times before losing power. In a graduate lab, I saw students run biodiesel synthesis using it, reporting cleaner product and less waste. That reduces chemical bills and bogs down less on waste disposal.
Extracting Metals and Biomolecules
Mining and pharmaceutical labs often run into headaches pulling specific metals or organic molecules out of complex mixtures. 1-octyl-3-methylimidazolium hydrogen sulfate grabs onto charged particles and organic compounds with both hands. Companies can pull rare earth metals from ore or separate plant alkaloids with higher yield and fewer byproducts. In practice, some researchers in India and China reported extracting uranium cleaner and faster than with traditional acids.
How to Keep the Benefits Without New Pains
Every chemical comes with tradeoffs. Some ionic liquids break down into toxic byproducts or cost a fortune to make at industrial scale. It takes careful screening to make sure a cleaner chemistry solution truly does less harm. For 1-octyl-3-methylimidazolium hydrogen sulfate, big improvements come from recycling and controlled disposal. Investing in closed systems and proper staff training reduces spill risks. Researchers can tailor the anion and cation to tweak features, like lowering toxicity or improving recyclability, so the next generation fits even better with environmental goals.
What’s Next in Sustainable Chemistry
Lab experience shows new chemicals stay popular only when they make life easier and safer. It pays to put effort into greener processes and waste reduction. No single solution fits every lab or factory, but the growth of ionic liquids such as 1-octyl-3-methylimidazolium hydrogen sulfate shows what direction modern chemistry is following: steady progress, safer workplaces, and a smaller mark on nature. Following the science closely and sharing results openly helps everyone keep raising the bar.
Getting the Chemical Formula Right
It’s easy to brush past chemical names, but every part matters. 1-Octyl-3-methylimidazolium hydrogen sulfate packs a lot into a single compound. The chemical formula for this compound is C12H24N2O4S. Breaking that down: C12 represents 12 carbon atoms, H24 stands for 24 hydrogens, N2 takes care of two nitrogens, O4 marks four oxygens, and S tags the single sulfur atom. These numbers don’t float on paper; accuracy pulls heavy weight in any discipline that touches chemistry.
I used to help a friend with their graduate lab work. More than once, we caught mistakes that started with a misplaced formula or a missed hydrogen here or there. Even the most careful researchers slip up if they rush this step. That’s why scientists double-check and triple-check these small details. Formulas left unchecked mean wasted chemicals, experiments that fall apart, or worse, sending wrong data onward.
Molecular Weight Can’t Hide
Every researcher uses a compound’s molecular weight as a building block. Run the math on C12H24N2O4S and you get a number: approximately 308.39 grams per mole. Anyone who’s prepped a sample for NMR or mixed up a reaction soup in a classroom or industrial lab knows that an off-target weight can turn an experiment sour. It's not just theory—costs stack up with each mistake, and time spent fixing those mistakes chips away at bigger goals.
I’ve seen firsthand how a miscalculation forced a club to cancel a whole afternoon of student experiments. There’s only so much grant money out there, and schools can’t flush it on do-overs. Having precise numbers helps keep budgets in check and sets the stage for safe, predictable reactions.
The Importance Hits Hard in Real Life
Ionic liquids like this one end up in labs looking for green chemistry solutions. These compounds don’t just sit on a shelf—they drive research with their unique properties. Scientists lean on ionic liquids to find less toxic, reusable solvents that reduce emissions in chemistry labs and the factory floor. If you want true “greener” chemistry, skipping details on formulas or weights only adds hurdles.
There’s also the regulatory and health side of things. A mistake in identifying what’s in your flask, or how much, sometimes lands as a compliance headache, or even a dangerous exposure event. Chemical handling falls under strict rules because mix-ups can have real-world consequences: spills, harmful fumes, or firefighting responses. Precision isn’t just for science—it’s a key part of keeping people safe.
Supporting Accuracy with Tools and Checks
Most labs use robust software and double-check processes—sometimes, it comes down to old-fashioned peer review. There are databases and calculators from trusted scientific groups. Cross-referencing sources like PubChem or Sigma-Aldrich helps prevent costly errors, and for beginners, even simple reference books become trusted allies.
In my experience, experience matters. The more you handle formulas and weights, the more you catch small slip-ups before they spiral. Mentoring others around this has always paid off, especially when new researchers join a team.
Moving Forward With Confidence
A future built on sustainable chemistry means keeping these basics airtight. As we work toward safer, smarter labs, remembering the basics—right down to individual carbons and hydrogens—protects experiments, budgets, and people.
A Closer Look at This Ionic Liquid
1-Octyl-3-methylimidazolium hydrogen sulfate belongs to the family of ionic liquids, those salt-like compounds which often stay liquid at room temperature. It’s used in plenty of laboratory and industrial settings, from catalyzing chemical reactions to extracting materials out of complex mixtures. The story goes that these liquids promise a greener future for chemistry because they aren’t flammable, hardly evaporate, and can sometimes be recycled. Still, before anyone hails them as the answer to clean chemistry, looking straight at their safety profile gives a reality check.
Workplace Risks and Skin Contact
I spent years in chemical labs, donning gloves, goggles, sometimes huffing under a poorly-fitted mask. Safety data sheets for 1-octyl-3-methylimidazolium hydrogen sulfate list it as an irritant—most direct skin contact leads to redness and itching, sometimes even a mild burn after long exposure. Once, a colleague accidentally splashed a tiny drop on her arm—after a quick rinse, a rash lingered for days. It sticks with you; chemicals that leave a mark after seconds should always raise a flag. Exposure limits for these newer ionic liquids rarely exist on national occupational health lists, but toxicologists consistently warn about prolonged or repeated exposure.
What We Know About Toxicity
Most toxicity studies on this compound come from animal research and cell cultures, not human trials. Chemicals with the imidazolium core show moderate toxicity in aquatic organisms—shrimp and fish exposed to typical concentrations struggle or die. One research group from Germany found that 1-octyl-3-methylimidazolium salts can cause cell membrane damage in aquatic species, mainly because this compound dissolves easily in water, slipping past cell barriers. That’s not just a threat to lab fish or shrimp; if industrial runoff heads into rivers, populations of important critters can drop.
For humans, oral toxicity looks fairly low, but nobody volunteers to drink it, so most risk comes from accidental spills or improper handling. Inhalation isn’t much of a problem since the stuff hardly vaporizes at room temperature. That offers some relief, especially remembering the sharp fumes from early organic solvents. Still, the cation—an imidazolium ring—has a reputation: longer alkyl chains often mean greater toxicity. Toxicity climbs as carbon chains stretch out, and 1-octyl is already at the upper limit used in most routine labs.
Handling and Disposal Realities
Disposal always gets less attention than handling, but ionic liquids tend to resist breaking down in nature. I’ve seen careless dumping of lesser-known chemicals into drains, and authorities in Europe already warn that many of these substances linger in soil and water, picking up where stubborn pollutants left off. Incineration under controlled settings remains the top method for disposal, but most labs don’t own that equipment. A sharp chemical manager refuses to let any significant volume of this stuff leave without a record.
Making Chemistry Safer
The idea of a “green” solvent needs some humility. 1-Octyl-3-methylimidazolium hydrogen sulfate tends to steer clear of open flames, but in the wrong hands, even “non-toxic” chemicals do harm. In my experience, risk drops fast if folks respect glove use, wear splash-proof goggles, and stick to closed systems. More real-world toxicity testing will help, but taking manufacturers’ safety advice seriously works just as well. No one compound solves pollution on its own, and believing ionic liquids are always safe sets you up for mistakes. Responsible storage, fire-safe cabinets, and regular training keep everyone honest. If anyone wants to use these chemicals for a new project, investing the time to read the safety sheet through and plan for smart disposal matters a lot more than any green marketing slogan ever could.
Grasping the Risks and Realities
1-Octyl-3-Methylimidazolium Hydrogen Sulfate stands out as an ionic liquid people use in labs and certain industrial settings. This compound does a good job as a solvent and sometimes finds its way into catalysis. Those who have spent any time in a busy lab know that proper treatment of chemicals prevents both small annoyances and dangerous accidents.
Storage Demands Respect
You won’t find shortcuts that pay off when stocking a shelf with this stuff. Leave the bottle open or fail to use a tight seal, and you invite water into your product. Moisture changes its chemistry and can spark unexpected reactions, even corroding metal shelves or equipment. Humidity control makes a difference. Don’t toss the bottle near a window or leave it on a bench by the sink.
Temperatures swing in some labs. Place this liquid on a shelf above a radiator or leave it in sunlight, and you risk breaking it down or releasing fumes you don’t want in the air. Cool, dry storage, out of direct light, gave me fewer headaches and more reliable results every time.
Handling: No Place for Shortcuts
People sometimes slap on thin gloves or skip protective eyewear for routine chores. Ionic liquids like 1-Octyl-3-Methylimidazolium Hydrogen Sulfate call for full nitrile gloves and splash-proof goggles. Spills feel sticky and cling to skin, and there’s a risk for irritation and burns if you ignore precautions. I’ve seen rushed researchers suffer rashes or worse simply for skipping the right barrier.
Work in a ventilated area. A fume hood saves trouble if volatile byproducts or fumes crop up. Though ionic liquids often carry low vapor pressures, ingredients like hydrogen sulfate can still bite if mishandled. Safe habits don’t slow you down as much as an emergency room visit or afternoon lost to spill cleanup.
Clear Labeling and Segregation
Use a label that says more than just a chemical name—record the date received and note hazard information. Keep incompatible chemicals apart. Acids and bases near each other tempt disaster; hydrogen sulfate mixtures stored near oxidizers or reactive metals ask for the same. Store this compound in a designated closet or bin, away from casual foot traffic and lunch breaks.
If you’re not sure about a neighboring bottle, check compatibility charts or ask someone with experience. Like many, I once learned the hard way after discovering two strong acids side-by-side, sharing fumes. It pays to create simple routines for chemical separation.
Disposal Isn’t a Footnote
After enjoying the clean-up part of a synthesis, don’t dump waste in generic barrels or down the sink. Collected waste with 1-Octyl-3-Methylimidazolium Hydrogen Sulfate belongs in dedicated containers rated for corrosive liquids. Label these, arrange regular pick-up with a licensed agency, and keep a logbook. In one lab, we kept a spreadsheet of every handoff, right down to the last drop. Authorities appreciate that kind of responsibility—and so does your conscience.
Continuous Learning Prevents Mistakes
Nobody gets everything right on day one. Training and practice sharpen the routine. Watch someone who’s done it for years handle these materials—they give contamination and spills a wide berth, double-check labels, and never work distracted. Trade stories, share lessons, and never be afraid to ask for advice. Everyone gets home safe, and the job gets done right.
Not Just Another Lab Chemical
Many chemicals fade into the background, but a select few keep grabbing attention. 1-Octyl-3-Methylimidazolium Hydrogen Sulfate sounds like something you’d avoid spilling in the kitchen, but researchers and industry specialists keep finding new ways to put it to work. As someone who’s helped troubleshoot messy extractions and stubborn reaction mixtures, I can attest: this “ionic liquid” doesn’t sit on the shelf gathering dust.
Making Chemistry Cleaner
The push for greener chemistry goes beyond buzzwords, especially when new regulations target old solvents. In the past decade, I’ve seen a lot of labs switch over to ionic liquids like this one for tasks like extracting metals, catalyzing reactions, and dissolving tough compounds. 1-Octyl-3-Methylimidazolium Hydrogen Sulfate, with its low vapor pressure and high thermal stability, stands out among them. It cuts out volatile organic solvents, which means less gas-mask drama and fewer headaches for everyone downwind.
Real-World Metal Extraction
Metallurgists and researchers in waste management have a tough job. Traditional metal extraction techniques—think harsh acids and endless rinsing—produce a ton of waste. This ionic liquid steps in to dissolve rare earths, copper, or uranium from ore or recycled material. In one project I joined, the switch reduced both the chemical load and the total number of steps, saving time and repair bills for worn-out equipment. Its tunable chemical properties let technicians tailor each extraction, making recovery more selective.
Catalysis With Less Fuss
Old-school organic solvents often make reaction cleanups long and miserable. I’ve watched teams spend hours separating out product from mixtures that should have taken minutes. 1-Octyl-3-Methylimidazolium Hydrogen Sulfate helps as a solvent and a “support” for catalysts. In certain acid-catalyzed reactions, it replaces traditional mineral acids—speeding up reaction rates and dropping the number of unwanted byproducts. Researchers using it in the synthesis of biodiesel or esters don’t just praise the increased yield—they like not having to scrub glassware for ages after finishing up.
Cellulose and Biopolymer Processing
Trying to work with cellulose, which makes up plant cell walls, is like trying to dissolve concrete in water. Biopolymer research took a step forward when this ionic liquid came on the scene. Its ability to break down stubborn biomass opened the door for more sustainable plastics, fibers, and even biofuels. Instead of relying solely on petrochemicals, labs now dig into agricultural waste and overgrown weeds, helping cut down the environmental toll of traditional plastic production.
Room For Improvement
Applications keep evolving, but there are real challenges. Cost and reuse matter a lot. Teams need to find more effective ways to recover and recycle this ionic liquid after use. Companies making batteries or specialty materials could benefit from collaborations with academic labs, drawing on both industrial scale and fresh research ideas. With more pilot projects and published data on recycling techniques, big users will feel more confident betting on a relatively new chemical.
No Shortage of Opportunity
As regulations around industrial solvents tighten, chemicals like 1-Octyl-3-Methylimidazolium Hydrogen Sulfate offer an outstretched hand. From where I stand, its story is just getting started. People working with metals, polymers, or fine chemicals need cleaner, less hazardous tools. Unless another breakthrough comes out of left field, this ionic liquid isn’t going anywhere soon—and I’ll be glad to see it on more benches, in more battered glass bottles.