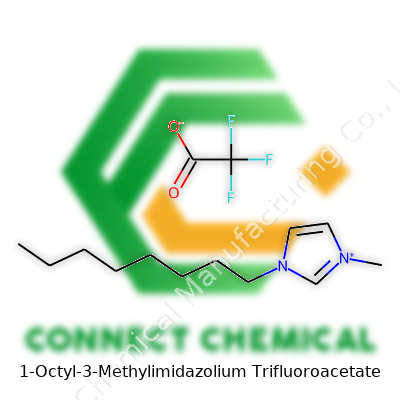
1-Octyl-3-Methylimidazolium Trifluoroacetate: Unpacking the Science and the Story
Historical Development
Back in the push for greener solvents, researchers kept stumbling against the limitations and risks of volatile organics. In the late 1990s, imidazolium-based ionic liquids caught attention for dissolving tough substances like cellulose, something regular solvents just shrugged at. The birth of 1-Octyl-3-Methylimidazolium Trifluoroacetate marked a turning point. This compound, which some call [OMIM][TFA], gave chemists a tool with powerful solubilizing abilities. As research into ionic liquids boomed across Europe, the US, and Asia, this substance stood out because its trifluoroacetate anion partnered well with a non-bulky, moderately hydrophobic cation. It didn’t just show up in laboratories, but in patent filings and industrial innovation, showing the world that ionic liquids could push chemistry towards more sustainable practices without sacrificing punch.
Product Overview
1-Octyl-3-Methylimidazolium Trifluoroacetate, often labeled as OMIM TFA, belongs to a group of room-temperature ionic liquids (RTILs) known for their stability, high thermal resistance, and unique solvent properties. Sold in colorless or pale yellow liquids, this chemical doesn’t evaporate or flash off in the way traditional solvents do. The chemical structure packs a one-octyl tail off the imidazolium ring, fostering solubility for both polar and non-polar species. That dual nature means chemists prefer it where tough separations or stubborn solutes need wrangling, such as biomass processing or selective extraction tasks.
Physical & Chemical Properties
With a melting point below room temperature, OMIM TFA comes as a stable liquid. Its density usually hovers around 1.15 g/cm³ depending on purity and water content. The viscosity is moderate — not syrupy, but thicker than water, easing pipetting and mixing without making things sluggish. The trifluoroacetate anion boosts both water compatibility and chemical resilience, so the liquid shrugs off oxidation and keeps its cool near strong acids or bases. Due to the long octyl chain, OMIM TFA avoids rapid water mixing, sitting as a low-volatility phase with a faint, characteristic odor. Thermal stability often stretches above 200°C, letting it stay useful in demanding lab setups. Its ionic conductivity reaches moderate values, fitting energy storage research while supporting efficient phase transfer in organic syntheses.
Technical Specifications & Labeling
Sourcing OMIM TFA from trusted suppliers brings certificates of analysis listing purity levels above 98%. Water content rarely runs above 0.5% to keep the performance high in sensitive processes. Chemical labeling follows international hazard codes—usually GHS—identifying trifluoroacetate’s possible irritant behavior and encouraging safe handling. Bottles arrive in amber glass to block UV exposure, with tight seals keeping out air and humidity. Labels clearly state batch numbers, lot tracking, and supplier information. Safety data sheets spell out recommended storage (generally below 30°C, away from strong acids or oxidizers) and proper first aid—important for both regulatory compliance and daily practice.
Preparation Method
Manufacturers produce OMIM TFA in a two-step synthesis that shows the modern drive to cut waste and maximize yield. They begin with N-methylimidazole and 1-chlorooctane, carrying out an alkylation that yields 1-octyl-3-methylimidazolium chloride. This intermediate is then purified before metathesis with sodium trifluoroacetate. The chloride and trifluoroacetate ions trade places, leaving OMIM TFA in the organic layer. Rigorous washing removes salts, followed by drying under vacuum to strip away trace water. Industrial setups now collect process waters for recycling, reducing the environmental load tied to ionic liquid manufacture.
Chemical Reactions & Modifications
OMIM TFA responds predictably under heat, light, and a variety of reaction conditions. It resists breakdown by strong acids and bases, unlike many common salts. Its imidazolium core can be functionalized for catalysis, while the long-chain cation allows researchers to tweak hydrophobicity as needed. Chemists sometimes swap the trifluoroacetate for other anions, targeting custom solvent blends or optimizing for specific solubility challenges. In green chemistry, OMIM TFA serves as both solvent and reactant, simplifying reaction setups and sidestepping multi-solvent recipes. The robust ionic bond between cation and anion gives the substance a wide useful window for thermal and chemical manipulation.
Synonyms & Product Names
Chemistry tends to attract a menu of names for versatile compounds. The most common synonyms include 1-Octyl-3-methyl-1H-imidazol-3-ium trifluoroacetate, OMIM TFA, and 1-Octyl-3-methylimidazolium trifluoroacetate ionic liquid. Some catalogs use abbreviations like [OMIM][TFA] or C8mimTFA, and research papers introduce minor tweaks based on local naming styles. No matter what it’s called, the compound retains the same core reactivity and risk, making it important to double-check synonyms during searches to avoid duplicate purchasing or data confusion.
Safety & Operational Standards
Safety handling remains front and center for anyone using OMIM TFA. Despite its appearance, ionic liquids require gloves, goggles, and good ventilation because the trifluoroacetate anion can cause skin and respiratory irritation. Although it doesn’t evaporate quickly, spills still demand immediate cleanup using absorbents and sealed waste containers. Laboratories enforce strict protocols for storage, separation from incompatible chemicals, and personal decontamination in the event of splashes. Regular training ensures that users understand the risks of improper heating, mixing or waste disposal. Responsible suppliers support this by providing detailed handling guides, batch certifications, and rapid customer support when safety questions arise.
Application Area
OMIM TFA now finds homes across a broad sweep of research and industry projects. It excels at dissolving lignocellulosic biomass, drawing out cellulose for bioplastic and biofuel breakthroughs. In the lab, researchers run catalytic reactions in OMIM TFA that deliver cleaner yields and reduce waste because the liquid can be reused. In separation science, it acts as a liquid-phase extractor, helping separate metal ions or purify rare earth elements without polluting water supplies. Electrochemistry groups employ it as an electrolyte in devices that push green energy innovation. Materials engineers look to OMIM TFA as an additive in polymer blends that require low volatility and thermal stability. Each application draws from the same core attributes—high stability, unique solvency, and resistance to breakdown in harsh conditions.
Research & Development
Research teams keep finding new ways to deploy OMIM TFA, particularly in growing fields like biorefining and advanced battery systems. Studies demonstrate that its tunable polarity and broad solubility give rise to simplified reaction mechanisms and better product purity. New catalyst designs now integrate OMIM TFA into the solvent matrix, squeezing more activity from metal centers while cutting catalyst leaching. A wave of publications digs into computational models of OMIM TFA, where its unique combination of hydrophobic and hydrophilic regions tells researchers a lot about how molecules interact in solution. Development efforts focus on scaling up green syntheses—an area ripe for partnerships between industry and universities eager to reduce waste and energy costs.
Toxicity Research
Nothing in chemical innovation moves forward without a look at toxicity. Over the last decade, labs subjected OMIM TFA to ecotoxicological and mammalian testing. Results showed low acute toxicity, but the trifluoroacetate component does raise questions about long-term environmental impact. Some studies noted bioaccumulation potential in aquatic environments, driving efforts to improve waste capture and recycling methods. Highly controlled disposal streams prevent accidental release, and industry guidance now calls for frequent audits of containment and recovery systems. Ongoing toxicology projects measure effects on enzyme activity and cell viability, supporting safe regulatory classifications across jurisdictions.
Future Prospects
OMIM TFA holds a spot in the broader movement to build greener platforms for chemistry, energy, and materials science. Innovation continues on less hazardous anions and biodegradable analogs, but the core structure remains a favorite as researchers chase bolder sustainability goals. Early tech-transfer projects involving OMIM TFA in closed-loop biorefineries already scale up pilot plants in Asia and North America. If new regulations push for detailed lifecycle analyses, OMIM TFA—designed for low volatility and reusability—offers a ready-made answer. The next chapter may see functionally engineered ionic liquids, with OMIM TFA at the center, supporting more efficient carbon capture, selective catalysis, and safer industrial processes.
Unlocking Biomass: Where Science Gets Practical
People often look at green chemistry and wonder if it's just a trend. It’s not. Take 1-octyl-3-methylimidazolium trifluoroacetate. For someone who has worked in both academic and industrial labs, tackling the sticky problem of breaking down plant biomass, new solvents that work well and minimize environmental impact quickly earn respect. Unlike old-school solvents that kick off fumes and flammable vapors, these ionic liquids—especially this one—change how we approach stubborn natural materials.
Cracking the Code of Wood and Grasses
Most natural plant matter is tough to break down. Lignocellulose in wood, straw, or even corn stalks puts up a fight, holding on to sugars and fibers with strong chemical bonds. Processors want the cellulose and hemicellulose inside, but typical acids and bases tear up the structure, then create more problems with waste or pollution. Here comes 1-octyl-3-methylimidazolium trifluoroacetate, which takes a more balanced approach.
In the lab, I’ve watched as this single ionic liquid quietly dissolved what strong acids couldn’t budge. It pulls apart that tight structure, leaving cellulose and other polysaccharides exposed and ready for further action—enzyme treatment, biofuel production, even creation of sustainable plastics. It works at moderate temperatures and keeps breakdown products to a minimum, which helps processors and engineers keep costs under control.
The process isn’t just cleaner, it’s more versatile. Researchers in the U.S., China, and Europe have all published on the use of this compound for enhanced saccharification—a fancy way to say turning plant stuff into fermentable sugars. Major companies and startups keep investing in biomass-to-ethanol projects, and the hope is to get off fossil fuels eventually. One piece to that puzzle is solvents like this one, which don’t gum up reactors or slow down yields with constant maintenance.
Challenges and Trade-offs
No chemical solves everything. Ionic liquids bring their own set of challenges. This molecule works well in labs and small-scale demos, but scaling up means watching for costs and making sure toxicity doesn’t get out of control. Waste handling can’t be ignored, either. The trifluoroacetate group can raise eyebrows if released untreated, so recovery and recycling are musts.
Still, compared with the mountains of solid waste and caustic runoff from classic treatments, I’d choose a system based on 1-octyl-3-methylimidazolium trifluoroacetate any day. Its selective solubility keeps more value in the final product stream instead of losing everything to degradation and contamination.
Building Toward Cleaner Chemistry
The ideal solution needs more than a single innovation. Real change comes from linking chemistry, engineering, and regulatory oversight. Success with this ionic liquid depends on building tough recycling systems, sharing data between factories and labs, and designing modern reactors that keep solvents contained while extracting as much value from renewable feedstocks as possible.
By following these steps, the world can turn agricultural and wood waste into fuels, chemicals, and materials without leaving as much environmental mess behind. The main application of 1-octyl-3-methylimidazolium trifluoroacetate isn’t just as another solvent—it’s part of a toolkit for moving toward greener industry. From where I stand, investing in these technologies gives us a better shot at sustainable living.
What This Chemical Means for Safety
Anyone who’s ever stepped into a chemistry lab quickly learns that not all liquids are equal. 1-Octyl-3-methylimidazolium trifluoroacetate is a mouthful, but it also lands on the radar for safety-minded folks. It's one of those ionic liquids you hear about in journals, often praised for its use in dissolving cellulose or as a solvent in green chemistry. That label "green" sometimes tricks people into letting their guard down, thinking less toxicity comes with every product aiming at environmental sustainability. In reality, a fancy name and a splash of “eco-friendly” don’t neutralize genuine hazards.
The Real Risks and Where They Show Up
You pick up a vial of this stuff, and you might not smell much—odors are generally faint. That doesn't mean danger isn’t around. Ionic liquids have a reputation for being less flammable, sometimes non-volatile, but not all toxicity hides under a boiling point. Some of them, including this one, can irritate skin or eyes if spilled or splashed. I came across a study out of Germany a couple of years ago that pointed out imidazolium-based ionic liquids often display moderate toxicity to aquatic life. Trifluoroacetate adds its own set of challenges: perfluorinated compounds don't break down easily once they escape into soil or groundwater. If wastewater treatment systems aren’t designed for these molecules, they can hang around for years.
Personal Protection and Best Practices
Throwing on gloves and goggles sounds like standard advice. In this case, it’s not just for show. Direct skin contact with imidazolium salts can cause redness or mild chemical burns over repeated exposure. Splashing into an eye leads to a day in the ER. Ventilation counts too, since accidental inhalation isn’t out of the question where fine mist or droplets form. I still remember an undergrad assistant forgetting his face mask while weighing out a similar liquid: a coughing fit and sore throat followed for half a day. Always take the extra minute to put on a lab coat and set up a fume hood, even if the bottle claims “low volatility.”
Waste and Environmental Impact
Lab workers and chemical engineers often underestimate the downstream effects. Sure, your bench looks clean, but flushing small quantities of trifluoroacetate-containing liquids down the drain builds up over time. Compared to standard salts like sodium chloride, these fluorinated anions resist breakdown—meaning water systems accumulate traces the longer labs ignore proper waste collection. These ionic liquids aren’t highly volatile, so they don’t evaporate into thin air. Their threat shifts toward water and soil, especially if disposal practices grow sloppy.
Finding a Safer Workflow
Plenty of research groups now look for biodegradable alternatives. Reading through journal articles from Japan and Scandinavia, I’ve noted an uptick in less persistent anions replacing trifluoroacetate. That said, if you still use 1-octyl-3-methylimidazolium trifluoroacetate, handle it in small batches. Use dedicated waste containers and call your hazardous waste service rather than disposing of it in regular lab trash. Rely on good storage—keep it tightly sealed, out of light, and away from acids or bases that might decompose it into nastier parts. Lastly, don’t wait for a regulator to tell you how to act safe—experience shows it pays off to treat every “unfamiliar” chemical as a potential hazard until you have enough good data and safe work protocols.
Why Smart Choices Matter
Safe habits in chemical labs don’t just protect individuals; they set standards for everyone else, from students to seasoned researchers. Getting careless with trifluoroacetate-based liquids could lead to health problems or environmental headaches for years to come. The responsibility for safety in the lab sits with each person who opens the bottle or signs the delivery log. Choose solid personal protection every time, treat waste as if it holds more than today’s inconvenience, and ask tough questions before introducing new chemicals into established workflows. That attitude keeps teams healthy—and the surrounding world safer.
The Building Blocks of 1-Octyl-3-Methylimidazolium Trifluoroacetate
Chemistry weaves together the world in quiet and powerful ways. Take 1-octyl-3-methylimidazolium trifluoroacetate, a compound that draws attention from researchers and industry alike. To start, the chemical structure combines two distinct pieces: the 1-octyl-3-methylimidazolium cation and the trifluoroacetate anion. The molecular formula for the cation forms as C12H23N2+. One side is a five-membered imidazolium ring; attach a methyl group at position three and an octyl chain at position one, and the result is a substantial organic cation. The anion, trifluoroacetate, steps in with the formula C2F3O2-; it’s acetate with three hydrogens swapped for fluorine atoms.
Lay the full chemical formula out, and it reads: C12H23N2 (for the cation) and C2F3O2 (for the anion), sometimes shown as [C8mim][TFA] for brevity in lab notes. The SMILES string, a language chemists lean on to share structures digitally, looks like: CCCCCCCCn1cc[n+](C)c1 for the cation, and CC(=O)[O-] with all the methyl hydrogens replaced by fluorines for the anion.
Why This Structure Matters
Ionic liquids, such as this compound, bring out a mix of oil-like behavior and ionic interaction. In my own experience working with solvents, ionic liquids often mean more flexibility in lab design. 1-octyl-3-methylimidazolium trifluoroacetate stands out because the imidazolium core offers chemical stability, and adding an octyl group makes the substance much less volatile. Safety in handling gets a big lift, which comes in handy if anyone has worked in crowded university labs where ventilation doesn't always match textbook ideals.
On the flipside, trifluoroacetate’s inclusion isn't just about making the name longer. Fluorinated compounds don’t mess around; they pack real punch in terms of stability and low nucleophilicity. Trifluoroacetate balances the organic cation, and this exchange builds an ionic liquid with a lower melting point, steady under a range of temperatures, and less flammable than many traditional solvents.
Real-World Impacts and Considerations
The importance of 1-octyl-3-methylimidazolium trifluoroacetate reaches into green chemistry. Lab work often faces sharp limits due to environmental hazards. Ionic liquids like this one can step in as alternatives to toxic, high-volatility solvents. The structure itself—bulky but manageable—acts as a scaffold for dissolving cellulose, extracting natural products, or even forming specialized reaction media for catalysis.
Yet, nothing in chemistry runs free from challenges. The presence of fluorine in trifluoroacetate brings up concerns about environmental persistence. I’ve seen a few studies track down traces in water that resisted breakdown. Any push for greener chemistry with this compound needs to look closely at waste management. Investing in better recovery and recycling technologies fits the bill. For instance, closed-loop systems can collect and reuse these ionic liquids, dropping the risk of accidental release and making their benefits more than just theoretical.
Looking Forward
The structure of 1-octyl-3-methylimidazolium trifluoroacetate—one heavy with promise, one heavy with responsibility—gives researchers a real shot at moving away from hazardous, outdated solvents. Careful stewardship and smart lab habits can open new directions for sustainable industrial chemistry, if everyone stays alert to both the potential and the limitations written into its structural formula.
The Realities of Working with Specialty Chemicals
Anyone who’s ever spent time in a chemical lab knows a careless approach leads straight to headaches and wasted resources. I learned early on to respect the quirks of ionic liquids like 1-Octyl-3-Methylimidazolium Trifluoroacetate. This isn’t a typical organic solvent to toss onto an open shelf. Improper handling quickly ruins purity, and sometimes causes safety risks that don’t show up on a safety data sheet.
Why Moisture Ruins More Than Your Lab Mood
Humidity can sneak up on you. I’ve watched traces of water creep their way into storage containers and ruin months of planning. Water shifts the behavior of this ionic liquid, changing viscosity and making it poor for tasks that demand reliable performance. For some researchers, that means wasted time purifying or even restarting entire batches. Best practice always keeps the bottle tightly sealed, using bottles that don’t let atmospheric moisture slip through. A desiccator cabinet, kept in a well-ventilated chemical storage area, works far better than hoping a regular jar lid does the job.
What Heat Does Behind Closed Doors
Every year, someone sticks a bottle on a windowsill or near a heat source. That spells unnecessary trouble. High temperatures break down these specialty salts. Expect off-odors, decomposition, or discoloration if it’s left out. Factory labels sometimes downplay temperature risks, but most experts I trust keep their 1-Octyl-3-Methylimidazolium Trifluoroacetate at or below room temperature—ideally around 20°C. In hot climates, chemical fridges provide peace of mind. Putting that container next to radiators or under bright lab lights is asking for an expensive replacement order.
The Cleanliness Factor Can’t Be Ignored
Small particles, dust, and stray chemicals find their way into every lab. Every time the container opens, the odds of contamination jump. I’ve watched teams reach repeatedly for the same spatula, only to introduce impurities they never considered. Using proper pipettes—clean and dry each time—preserves the chemical’s integrity. It sounds simple, but forgetting this step shortens shelf life and compromises results. Labeling each bottle and using dedicated tools avoids guessing games during cleanup or audits down the line.
Ventilation Isn’t Just for Smelly Reagents
Some people skip proper ventilation because 1-Octyl-3-Methylimidazolium Trifluoroacetate doesn’t throw off a strong scent. That gets risky if the bottle tips or leaks. These fluorinated trifluoroacetates possess chemical reactivity that doesn’t play nice with skin, eyes, or lungs. A spill, even if minor, should motivate everyone to use a proper fume hood for storage. This protects not only product quality but also prevents that unpleasant surprise if the substance reacts with other materials left on an open bench.
Labeling and Inventory—The Stress-Free Approach
Watching coworkers scramble to find out whether a bottle’s contents expired or not still gives me flashbacks. I mark each bottle with the date received and the date opened. Routine checks catch degradation early, especially for rarely used chemicals. Digital inventory logs help larger research labs track storage and cut down on panic when supply chains run slow or student assistants come and go. Good documentation wins every time over guesswork.
Final thought: Storing chemicals like 1-Octyl-3-Methylimidazolium Trifluoroacetate isn’t complicated if you treat the task with respect and attention to details lab veterans swear by. Small habits save bigger headaches.Understanding Purity Isn’t Just for Scientists
Anyone in manufacturing, healthcare, or even food industries knows one thing: what’s in the container matters just as much as what’s written on the label. Purity, or the concentration of the main ingredient, isn’t just a technical detail—it directly impacts safety, quality, and even the price.
Purity: More Than a Number
A lot of people outside the lab overlook the ripple effects of low purity. Think of a pharmaceutical ingredient. If the active part isn’t strong enough, the final medicine might fail to do its job. I’ve seen a batch of vitamin supplements marked “pure” but actually loaded with fillers. People spent money expecting real nutritional value and got something much weaker. Confidence drops, sales drop, and worst of all, customers lose their trust.
Manufacturers can’t take purity at face value. They need a certificate of analysis from their supplier. This piece of paper should spell out exact concentration and any unwanted substances. Sometimes, buying in bulk at a low price brings a false sense of security—hidden impurities cost plenty when products don’t meet regulations or get flagged during audits.
Real-World Impact
Purity affects the environment too. Lower purity often means using more raw material to get the right outcome. In agriculture, for example, impure fertilizers require higher quantities to be effective. That ends up costing more in the long run—extra transport, more emissions, and extra waste disposal. On top of this, if a supplier isn’t transparent about product makeup, dangerous contaminants can slip through to the farm, kitchen, or factory floor.
Checking Before Buying
No business should settle for vague product sheets. I always ask for third-party test results and detailed specifications. Product data sheets and safety documents help, but independent laboratory results tell the real story. I’ve learned not to rely on casual promises from sales staff. Direct questions bring out the truth: what percent is the actual ingredient? What else is present in even the tiniest amount?
Mistakes in this area get expensive. In construction, for example, if concrete-additives are less concentrated, entire batches of concrete end up weaker or cure the wrong way. Rebuilding isn’t cheap. It only takes one shortcut to ruin an entire project.
Solutions That Actually Work
Companies serious about quality always invest in relationships with reputable suppliers. They set up regular audits and random batch testing. Some bring in third-party labs to verify shipments before products even enter inventory. Training purchase staff to know the right questions saves headaches later. Open communication between buyers and suppliers helps root out bad practices quickly.
Governments and industry groups have a job here too. Setting clear guidelines about minimum purity or concentration puts everyone on the same playing field. Penalties for misleading claims need to hurt enough to deter cutting corners. Public databases of non-compliant suppliers make shady dealing a lot riskier.
Quality comes from clear standards, strong oversight, and honest supply chains. The stakes touch health, budgets, reputations, and even the planet. Purity isn’t just a tech term—it’s something buyers, sellers, and end-users all should care about, every time.