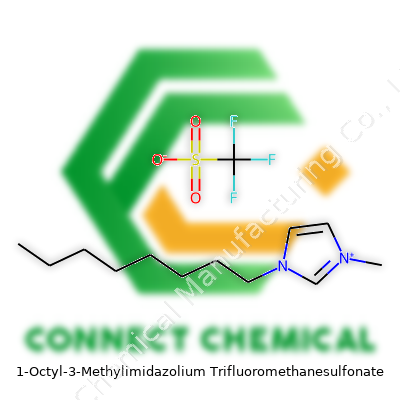
1-Octyl-3-Methylimidazolium Trifluoromethanesulfonate: A Deep Dive
Historical Development
For years, chemists chased after better ways to break free from traditional solvents. The pursuit of ionic liquids gained steam during the 1990s, as researchers grew weary of handling volatile organics in stuffy labs. Out of this push, imidazolium-based salts like 1-Octyl-3-methylimidazolium trifluoromethanesulfonate carved out their place. Not because anybody handed them the trophy, but because they offered practical relief from flammable vapors and waste, with early papers showing potential for less evaporation and better recycling. Countless studies by pioneers such as Wilkes and Seddon pointed to unique behaviors of these ionic compounds, drawing the interest of both academics and greener process engineers.
Product Overview
The compound stands as an ionic liquid: a salt staying liquid below 100°C, typically at room temperature. Its imidazolium ring acts as the heart, while the octyl tail and methyl group set it apart from shorter-chain siblings. Add the trifluoromethanesulfonate anion, famous for resisting breakdown in harsh systems, and you get a liquid that doesn’t just sit in the bottle but gets into real-world applications. Typically, it appears pale yellow and slightly viscous, catching light in a way that signals its density. Some supplies carry minor impurities from synthesis or handling; reputable sources document content and purity in detail, offering 99% or higher for precise research.
Physical & Chemical Properties
On the bench, 1-Octyl-3-methylimidazolium trifluoromethanesulfonate reveals a weighty presence. Its melting point hovers well below human comfort, slipping into liquid form below 30°C. Specific gravity closes in on 1.2, meaning it’s heavier than water but easy to manipulate with pipettes or syringes. Viscosity lands in the medium-to-high realm, especially as temperatures drop, meaning laboratory handling sometimes takes patience or gentle warming. Water loves to sneak in, affecting conductivity and solubility—the compound’s strong affinity for moisture stems from its polar groups, prompting storage under dry, nitrogen or argon conditions. Chemically, it holds up against basic and acidic solutions, avoiding hydrolysis or breakdown even with sustained heating. Stability against light and oxygen lets it survive on open benches longer than most organics, though sealed containers outlast open ones.
Technical Specifications & Labeling
Every reputable bottle should state molecular formula C12H21F3N2O3S and a molecular weight near 346.37 g/mol. Safety labeling reveals sensitivity to both eye and skin contact, requiring gloves and sealed glasses. MSDS information often flags potential long-term hazards, pointing to limited but noteworthy health studies. Container labels tend toward clear hazard pictograms, often paired with batch numbers and manufacturer’s certification of purity. Some manufacturers add certificates of analysis—documenting water content, halide contamination, and major ion chromatography results—reflecting just how seriously researchers take trace impurity control.
Preparation Method
Synthesis doesn’t call for impossible glassware, but workers respect its demands. Alkylation of methylimidazole with 1-chlorooctane sparks the base structure, producing the imidazolium chloride salt. That product enters metathesis with sodium—or silver—trifluoromethanesulfonate, typically run in dry solvent under nitrogen at controlled temperatures. Filtration and washing remove trace halides, while rotary evaporation and vacuum stripping chase out residual solvents. More significant syntheses pour through neutral alumina or ion-exchange columns, squeezing out unwanted ions that would trip up sensitive measurements. Some labs even pass product through activated charcoal to clear up color and odorous byproducts before careful bottle transfer and final nitrogen blanketing.
Chemical Reactions & Modifications
Though tough, 1-Octyl-3-methylimidazolium trifluoromethanesulfonate’s structure isn’t immune to smart tweaking. Substituting the octyl group for shorter or bulkier chains shifts solubility and viscosity, while swapping the trifluoromethanesulfonate anion unlocks new properties. Its cation resists nucleophilic attack, sidestepping side reactions common in older solvents. Reactivity stays low with transition metals, but clever catalysis enables transfer hydrogenation, Sonogashira couplings, and even green oxidation—all done more sustainably compared to volatile organic solvents. Chiral separation and selective extraction also become practical by combining the ionic liquid with tailored additives. Research still uncovers new synergies, hinting at catalytic futures unimagined a decade ago.
Synonyms & Product Names
Don’t expect clarity in trade names. Some label this ionic liquid as OMIM TfO, 1-Octyl-3-methylimidazolium triflate, or just [OMIM][OTf] in shorthand. Alternative suppliers stamp their own product numbers, sowing confusion among newcomers, but standard IUPAC tags now dominate formal literature. Searching patent filings, you’ll sometimes spot names like 1-methyl-3-octylimidazolium trifluoromethanesulfonate—spelling out everything for legal clarity. For practical purposes, cross-checking CAS Numbers—usually 393108-66-8—sidesteps mix-ups, especially when working across borders or sourcing for regulatory reasons.
Safety & Operational Standards
Handling leaks and spills demands more than casual care. Workers wear gloves and avoid inhalation, especially in poorly ventilated places. Small spills get treated with absorbent pads followed by solvent rinses, preferably with only trained staff present. Storage runs best in tight, amber glass under dry, inert atmosphere to limit oxidation or water uptake. Local codes frequently treat it as an industrial chemical, not a benign household product, reflecting safety incidents tied to improper transport and handling. Regular training lowers mishaps, while spill kits and eyewash stations serve as backstops for the rare emergency.
Application Area
Applications of 1-Octyl-3-methylimidazolium trifluoromethanesulfonate pop up across multiple industries. Chemists reach for it to boost efficiency in organic synthesis and extraction, its ionic structure dissolving a wild range of organics and inorganics. Green engineering teams tap its non-volatility for solvent recycling, shifting away from wasteful evaporation. Electrochemists turn it into electrolytes for batteries and capacitors, counting on its wide electrochemical window to handle high-voltage experiments without decomposing. Lubricant makers value its stability at temperatures regular oils couldn’t touch, using it in some specialty greases and anti-wear coatings. Analysts lean on it as a solvent for NMR, especially for hydrophobic compounds, skipping the troubles that come with water or traditional solvents. Research reports even suggest potential as a corrosion inhibitor in tough industrial environments, with the triflate anion tuning interface chemistry for stubborn materials like stainless steel.
Research & Development
Current research pushes into new territory. Scientists test its limits in extracting rare earth elements and heavy metals, hoping for cleaner and more selective processes in e-waste recycling and mining. Polymer chemistry innovators experiment with embedding it in membranes and coatings, gaining fresh control over permeability, selectivity, and resistance. Green chemistry labs run it as a reaction medium for metal-catalyzed cross-couplings, measuring yield and selectivity for emerging pharmaceuticals. Hybrid materials—combinations of this ionic liquid with nanomaterials or enzymes—keep showing up at conferences as novel candidates for advanced sensors, membrane systems, and energy storage. Ongoing efforts seek to uncover why certain cation-anion pairs outperform others in tricky reactions, while computational chemists model these liquids at atomistic scales to untangle complex ionic networks and predict physical traits before anyone breaks out the glassware.
Toxicity Research
Toxicity stories so far reflect caution, not alarm. Lab animal studies show the cation’s long alkyl chain can disrupt cellular membranes, while the anion’s persistence in water environments raises questions about long-term breakdown. Few chronic exposure studies yet exist, but the ionic nature complicates removal in traditional water treatment, meaning labs and factories can’t treat it as harmless drain waste. Regulatory agencies still watch reports of aquatic impacts, with researchers evaluating its effects on algae, invertebrates, and fish. Standard practice calls for secure waste collection and licensed disposal, since tradition teaches that today’s miracle solvent too often carries tomorrow’s legacy risks.
Future Prospects
Prospects for this ionic liquid stretch well beyond its current uses. As battery makers scramble for safer, higher-performing electrolytes, demand for robust ionic conductors keeps rising. Green manufacturing pushes drive deeper substitution of problematic organics—in pharmaceuticals, fine chemicals, and renewables—with process chemists looking to scale up without inviting fire marshals to every meeting. Waste-cleanup researchers eye its use in removing persistent pollutants, focusing on regeneration to keep costs manageable. Synthetic biologists imagine pairing it with engineered enzymes to tackle stubborn feedstocks. Sustained investment in greener, more biodegradable analogs hinges on regulatory acceptance and price drops as production scales mature. As science digs deeper, the simple utility of 1-Octyl-3-methylimidazolium trifluoromethanesulfonate touches an ever-broader band of chemical innovation.
From Green Chemistry Labs to Industrial Processes
Walking through a modern chemistry lab, I see 1-octyl-3-methylimidazolium trifluoromethanesulfonate—some know it as an ionic liquid—tucked in with other specialty solvents. I recognize its unique place in the push for greener, safer alternatives to volatile organic solvents. This salt keeps its liquid state at room temperature and doesn’t evaporate easily, which cuts down on exposure to toxic fumes during research or production. Many researchers pick it when a process should run with minimal emissions or fire risk. That’s not just good practice, it’s smart science.
Electrochemistry and Batteries: Chasing Better Power Sources
Tinkering with energy storage has forced the field to step up its game. I’ve talked to engineers who praise this ionic liquid for its remarkable electrochemical stability. Its large, awkward structure keeps current flowing without breaking down as fast as water or traditional electrolyte mixes. Industries aiming for high-performance supercapacitors or lithium-ion batteries look at this salt with keen interest. It tolerates higher voltages and doesn’t let moisture crash the party, which means devices can last longer and face fewer surprises under heavy use. Keeping electronics safer and our environment a little cleaner goes hand-in-hand here.
Catalysis: Cleaner Routes to Products We Use Every Day
Transforming raw chemicals into dyes, flavors, or medicines usually calls for a catalyst—and ionic liquids are becoming a top pick for those recipes. In my own work, I’ve used this imidazolium-based liquid to boost reaction speeds and bump up yield. Its ability to dissolve both organic and inorganic compounds gives researchers lots of flexibility. By cutting out harsh solvents or temperamental catalysts, chemists reduce waste and often end up with reactions that are easier to manage and scale. The job becomes about precision and control, not just brute force and hope.
Separation Science: Cleaner Streams, Safer Water
Anyone dealing with environmental testing can vouch for the frustration in picking the right extraction agents. Triflate-based ionic liquids show up as strong players for pulling out trace metals, stubborn dyes, or pharmaceutical leftovers from water samples and industrial waste. I’ve seen more labs choose them because they’re stable and don’t mix into water—making cleanup and recycling a breeze. Regulators also prefer methods that don’t dump new hazards into already sensitive samples, which makes these salts an appealing solution.
Potential Solutions and Future Steps
Improving the cost profile sits at the core of wider adoption. Large-scale use demands production ramps and methods to reclaim and recycle the ionic liquid after each use. Researchers and manufacturers can join forces, sharing best practices for closed-loop systems. Funding agencies could nudge the field toward greener, recyclable formulations by backing projects that repurpose used material.
Even as industries search out winning materials for green chemistry, none deliver overnight magic. With each new application of 1-octyl-3-methylimidazolium trifluoromethanesulfonate, the challenges of cost, scale, and safety point the way to smarter questions—and better materials for a world that cares about every drop and every breath.
Purity: More Than Just a Marketing Number
Questions about purity pop up all the time, especially if you’re dealing with products like supplements, chemicals, or even food ingredients. Purity level isn’t just something to catch your eye on a label—it tells you a lot about what you're putting your trust in. Seeing “99% pure” looks impressive. Still, that last percent holds real weight, whether you're a baker, a scientist, or just health-conscious. As someone who’s worked in both a university lab and a small food business, I’ve seen up close why purity matters so much.
Personal Experience in the Lab and Beyond
A few years ago, I helped a professor run basic experiments on vitamin C powders. One brand claimed pharmaceutical-grade quality at 99.9% purity, while a cheaper option came in at about 95%. On paper, a 4.9% difference doesn’t seem huge. But step into the lab, and that small percent can mean extra sugars, fillers, or even trace contaminants. This translates to changes in color, taste, and—most importantly—safety, especially when you’re scaling up for food production or clinical use. Even outside pure science, this same principle hits home for small businesses selling spices or health products.
Trust—Backed by Testing, Not Just Labels
Labels can promise a lot, but they don’t guarantee what’s inside. To back up purity claims, many reputable producers offer certificates of analysis from independent labs. These reports tell the whole story—what methods were used to analyze the product, what impurities got flagged, and at what levels.
If a company doesn’t have lab data ready, that’s suspicious. In my own kitchen business, we always asked for third-party testing reports when sourcing vanilla and baking soda. Trace impurities, such as heavy metals, aren’t just regulatory hurdles—they’re a health risk. Documented outbreaks linked to supplements and powders that skipped honest testing keep cropping up. Just last year, the FDA caught several supplement brands selling "pure" products that contained undeclared drugs and contaminants. That’s the kind of news that shakes consumer confidence at a deep level.
Purity and Price: A Tough Balance
High purity usually costs more. The expense comes from more filtering, slower processing, and stricter quality controls. For families or small manufacturers, the budget just won’t stretch to the highest levels every time. Price can push buyers toward products with lower declared purity or brands that can’t afford routine testing. It’s a tough reality I’ve seen in local markets, where budget-conscious customers rely on trust in the local shopkeeper rather than lab reports.
Making Sure Purity Claims Stand Up
Reliable purity means more than a bold font or shiny label. It calls for companies to share their data, answer questions about testing, and treat consumer concerns seriously. As buyers, asking for documentation helps push everyone toward safer, cleaner products. Big or small, every producer owes their customers honesty about what's inside. The next time you see a purity percentage, remember there’s more behind it than a number. This is about trust, health, and shared responsibility.
Caring for What We Store
Too often, we sling groceries or medicine into a cabinet or closet, thinking a closed door gives enough protection. Truth is, most things—food, medicine, chemicals—demand more attention. Every box, bottle, or bag can lose its value, safety, or flavor unless the right conditions are part of the picture.
Temperature and What It Changes
On a hot summer day, chocolate melts right in your hand. That same heat can spoil vitamins and medicine, too. Temperature swings stress chemical bonds, break down active ingredients, and turn yogurt from healthy snack to science experiment. Decent refrigerators work well for dairy, some vaccines, many kinds of fruit, and leftover dinner. Pantry items like rice and canned soup sit comfortably in the low 20s Celsius. Pfizer’s COVID-19 vaccine changed the conversation when it showed up with a -70°C requirement—without that chill, batches lost their punch.
Food safety experts say that the “danger zone” for bacteria lies between 4°C and 60°C. That means perishable food deserves a quick trip into a fridge, not a slow death on the counter. Over years of working in restaurants, I learned to trust the walk-in cooler more than any fancy coating or “best by” sticker.
Moisture—The Silent Enemy
Bread turns stale on dry days and grows green fuzz when it stays humid long enough. Most grains and flours, even electronics, keep their best quality in dry spaces—below 60% relative humidity works well for much of what we buy. High humidity triggers mold in flour jars, corrosion on phone circuit boards, and clumps in powdered milk. Keeping silica gel packets in electronics cases or tossing a paper towel in a salad spinner both keep air drier and goods fresher.
Light—Unseen Damage
Some over-the-counter pills and vitamins break down with too much light. My grandmother used to keep medicine bottles in a dark drawer for a reason. Bright light or sunlight heats up surfaces, fades labels, and causes vitamin C and B2 to lose effectiveness. Opaque or amber bottles and pharmacy bags block much of this. Sunlight ruins both pigment in paints and certain canned foods, so keeping these out of window glare helps preserve their color and nutritional value.
Safe, Clean, Well-Labeled
Whether you’re storing coffee or chemicals, putting them somewhere clean, clearly labeled, and away from children means fewer accidents. Over my years of working in a hospital lab, locked cabinets and organized labels saved us from mixing up reagents and kept us ready for inspections. For homes, sealed bins help keep out mice and bugs, and regular wipe-downs keep spaces free of spills and residue.
Common Sense Wins
Most of us don’t have time to overthink every bag of flour or jar of pickles. But paying attention to temperature, moisture, light, and clear labeling makes life safer and keeps money in our pockets. Reading the label, asking a pharmacist, or checking a reliable government food safety site always brings more trustworthy advice than guesswork. Taking simple care and using a bit of experience makes everything last longer—whether it’s lunch for tomorrow or medicine for the week.
Clearer Health Risks
Some chemicals carry dangers that paperwork can’t cover with a single safety symbol. I’ve worked in labs where even the stuff marked as “common” called for special gloves, fans running nonstop, and a healthy dose of respect. Strong acids, volatile solvents, and seemingly simple powders like silica dust all turned out riskier than I realized. Read the label, yes, but also dig into the safety data sheet (SDS). The SDS breaks down possible impacts, whether you breathe it in, touch the powder, or accidentally spill it on a bench.
Most people don’t walk around thinking about respiratory risk or skin absorption. Benzene—just as an example—used to be seen as just another solvent. Over time, stronger regulations grew out of research connecting benzene to leukemia. It taught many of us that what seems routine might silently threaten health. OSHA, NIOSH, and European safety agencies have long lists of compounds under special scrutiny for exactly that reason.
Don’t Trust Comfort
Everything looks easy until it doesn’t. Bleach makes a regular appearance in kitchens and laundries, but it’s caustic, and inhaling its fumes mixes strangely with ammonia from common cleaners. In the best scenario, you’re left with chest tightness; in the worst, you’re calling poison control. Household fixes can go wrong with a single careless combo. It’s a reminder: just because something smells familiar doesn’t mean it’s harmless.
Out in industry, even a touch of lead or mercury on the skin equals months of chronic exposure risk. Despite training, slip-ups happen. I remember a workplace that used phenol—a liquid with a deceptively sweet scent. Even a fingertip soaked in phenol could become a medical emergency. The way to avoid it isn’t just “handle with care”; it means wearing the right gloves, checking ventilation, and knowing that soap and water aren’t always enough.
What Informs Better Handling?
Most mishaps come from shortcuts. Regulation exists because people paid the price before guidelines appeared. Over 40,000 chemicals see daily use in U.S. commerce; not all of them show their teeth right away. “Special handling” means specific gear (think goggles, not sunglasses), good room airflow, and sometimes storing materials away from sunlight or water. Fact: some chemicals react to the moisture in air, which has led to explosions or runaway fires. Considering the tragic events in Bhopal, India, with methyl isocyanate—resulting in thousands of deaths—industry now holds emergency drills and keeps stricter inventory tracking.
Looking Ahead
Learning isn’t just memorizing which compound matches which hazard symbol. It’s understanding exposure doesn’t happen only in big factories. Schools, garages, and farms keep plenty of risky materials close at hand. Asking, “Do I need goggles?” is no longer a sign of inexperience; it’s part of keeping your home, family, or workplace safe.
Solutions start with training—regular reviews, not a single meeting when a chemical first appears on site. Labeling should be clear and permanently attached, not scratched by years in storage. Emergency showers and eyewash stations need to function, and spill cleanup kits should be close by, not locked in a distant storage closet. Mistakes come from routine. Real safety comes from measured respect for every compound that could hurt you if given the chance.
Understanding Its Identity
Chemists run into a dizzying array of names and numbers, especially with ionic liquids changing how research and industry solve real challenges. 1-Octyl-3-methylimidazolium trifluoromethanesulfonate stands out for its unique combination of properties. The CAS number for this compound is 271717-86-7. Its molecular formula reads C13H23F3N2O3S. That’s more than some chemical trivia. It’s a handle for tracking purity, safety, sourcing, and legal compliance, whether we’re reading a safety sheet or ordering from a supplier.
Real-World Benefits and Challenges
What drew my attention to this compound wasn’t just the tongue-twister name. It’s how ionic liquids like 1-Octyl-3-methylimidazolium trifluoromethanesulfonate promise less pollution compared to traditional organic solvents. Volatile, flammable solvents have caused headaches for decades, both in workplace safety and environmental cleanup. This ionic liquid, made of a bulky organic cation and a non-coordinating anion, almost refuses to vaporize. That means less risk of inhalation and lower fire hazards in labs and factories.
In my own experience, tackling a difficult extraction task with conventional solvents can raise exposure risks and disposal hassles. Swapping those for an ionic liquid feels like finding a shortcut through a traffic jam. Even purifying compounds from stubborn matrices gets easier, often at lower temperatures, cutting energy bills. Plus, ionic liquids like this one have roles in catalysis, battery technology, and even carbon dioxide capture — fields that matter for greener chemistry and next-generation materials.
The Need for Clear Identification
College chemistry labs teach you to treat every unknown vial like an accident waiting to happen. On the other hand, having a clear molecular formula and CAS number instantly unlocks databases, hazard sheets, and regulatory documents. For 1-Octyl-3-methylimidazolium trifluoromethanesulfonate (CAS 271717-86-7), this accuracy helps avoid dangerous mix-ups, enables efficient procurement, and supports transparent reporting. Those numbers help a new researcher or a regulatory inspector get the same facts — a foundation for safe, effective lab practice.
Addressing Industry and Environmental Concerns
The rise of ionic liquids highlights both promise and growing pains. Even with low volatility, some ionic liquids have shown unexpected persistence in soil and water. Simple substitution isn’t a magic bullet. Researchers, myself included, keep an eye on long-term toxicity data, environmental fate, and lifecycle impacts. Not every “green solvent” lives up to the hype once it enters the wastewater stream. Better analytical techniques and cross-disciplinary partnerships can drive smarter choices, making sure these advances don’t introduce hidden risks.
Companies and academic labs often face pressure to innovate sustainably without enough data on new materials. I see colleagues pushing for more open-source testing and shared databases. Communicating hazard data, test results, and real-world performance may take extra effort, but it gives everyone — from lab techs to regulatory officers — a fighting chance to weigh both innovation and long-term safety.
Moving Toward Smarter Chemistry
Every new material brings hopeful progress and new questions. Choosing ionic liquids like 1-Octyl-3-methylimidazolium trifluoromethanesulfonate for specific tasks can push both industry and research toward a cleaner future, but only if scientists, managers, and regulators keep experience and evidence at the forefront. Open communication, persistent safety checks, and honest assessment of both benefits and risks push chemistry forward without losing sight of what matters most — people and the world we live in.