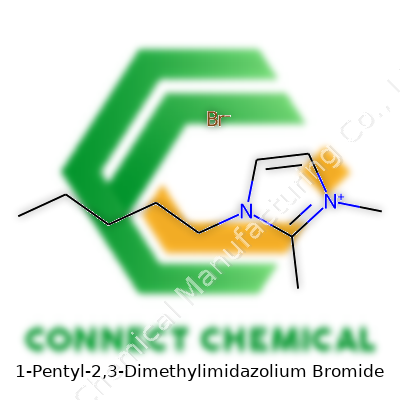
1-Pentyl-2,3-Dimethylimidazolium Bromide: A Down-to-Earth Commentary
Historical Development
Work with ionic liquids always drags along a history of slow discovery and stubborn experimentation. Chemists searching for new solvents in the late twentieth century hovered over the imidazolium backbone to push chemical boundaries. Substituents at every spot on the ring produced compounds with odd solubilities and tempers, and the pentyl-2,3-dimethyl variant didn’t just fall out of the sky. Research labs swapped methyls and alkyls hoping to improve melting points and chemical stability. It eventually caught the eye of researchers tweaking reaction media for improved catalysis or greener solvents. 1-Pentyl-2,3-dimethylimidazolium bromide stepped into synthetic labs as people searched for liquid salts with lower toxicity and stronger resilience than volatile organics. Its progress didn’t ramp up all at once—funding fluctuated, and the compound waited its turn to be taken up in real applications.
Product Overview
1-Pentyl-2,3-dimethylimidazolium bromide, often sold as a pale crystalline solid or viscous liquid, crosses lab benches as a relatively stable ionic liquid. Its signature comes from the pentyl chain and odd methyl placements along the imidazolium ring. The presence of the bromide anion brings certain solubility and conductivity perks not found in classic imidazolium salts. Research suppliers label it as a specialty chemical, packaging under dry conditions to prevent caking, namedrops like [1-Pentyl-2,3-dimethylimidazolium]Br or [PmMIm]Br landing in catalogues. Demands come mainly from academic research and parts of industry dabbling in solvents where classic choices don’t bring value anymore. Packaging ranges from small vials to bulk containers, always calling for clear labeling and quality control.
Physical & Chemical Properties
This compound does not try to hide its physical traits; you get a melting point that slides below 100°C, letting the substance flow as a room temperature ionic liquid under many conditions. It finds balance between hydrophobic and hydrophilic domains, thanks to the pentyl and methyl mix, pulling some solvents closer and pushing others away. The color sometimes tints from clear to pale yellow, depending on tiny impurities left from the synthesis. Density sits close to 1.1–1.2 g/cm³, heavier than water but less than other ionic liquids with bulkier ions. Conductivity fares well—enough for practical electrochemistry, since the ions move freely in the liquid state. Thermal stability holds up to moderate temperatures, though pushing above 200°C without decomposition tests anyone’s patience or lab setup. Solubility in water depends on the length and number of alkyl chains, so pentyl shifts the landscape compared to more familiar methyl and ethyl relatives.
Technical Specifications & Labeling
Vials leave certified labs with specifics: purity typically no less than 98%, water content below 0.5%, halide content tested batch-wise. Labels don’t just slap on a name—they spell out CAS numbers, hazard phrases, and storage recommendations. Safety data sheets run through regulatory checks, underlining measures for accidental exposure, spillage, or disposal. Certificate of analysis comes alongside each lot, giving buyers confidence in reproducibility and compliance. Every label must highlight the bromide anion, since halide content influences not only applications but also safety protocols and environmental impact in disposal.
Preparation Method
Making 1-pentyl-2,3-dimethylimidazolium bromide is no seat-of-the-pants operation. Start with 2,3-dimethylimidazole, react it with 1-bromopentane in solvent conditions that fit both safety and yield requirements. Strong stirring, controlled temperature—not too hot, not too cool—keeps side products at bay. After several hours, the reaction mixture gives way to the desired quaternized salt. Removing excess 1-bromopentane and solvents by rotary evaporation, then washing with ethyl acetate, sharpens the product’s purity. Crystals dry under vacuum to finish the compound in a usable state. Reaction steps sometimes vary by scale or local safety constraints, but the backbone of this process holds up across global labs.
Chemical Reactions & Modifications
Chemists hungry for custom ionic liquids use 1-pentyl-2,3-dimethylimidazolium bromide as a gateway to more complex salts. Metathesis reactions swap the bromide for other anions—PF6-, BF4-, NTf2-—using salt exchange protocols in water or organic layers, followed by careful washing to remove leftover impurities. The alkyl and methyl groups on the imidazolium ring sidestep harsh conditions, but strong nucleophiles or bases invite ring opening or demethylation, especially if temperature or concentration isn’t watched. Researchers looking for designer ionic liquids sometimes functionalize the pentyl chain, tacking on polar groups or unsaturations by selective reactions with mild reagents. Those pursuing greener chemistry stay wary of halide residues and unintended byproducts.
Synonyms & Product Names
On inventory sheets and research papers, this compound turns up under different code names: 1-Pentyl-2,3-dimethylimidazolium bromide, [PmMIm]Br, and in chemical registry shorthand as C10H19N2Br. Vendors offer it as a “custom” or “research” ionic liquid, marking it apart from the run-of-the-mill butyl or methyl imidazolium salts. Different catalogs sometimes shift the order of substituents or abbreviate, but the structure never lies. Buyers need to scan label information and chemical structure diagrams to double-check compatibility before launching experiments or scale-up efforts.
Safety & Operational Standards
Handle with respect, like most ionic liquids containing halide anions. Gloves, goggles, and good ventilation keep the odds in favor of lab workers. 1-Pentyl-2,3-dimethylimidazolium bromide brings modest toxicity—skin or eye contact isn’t catastrophic, though it can irritate. Inhalation risk stays low at room temperature, but heating or mishandling can aerosolize particles or release decomposition products. Environmental authorities recommend limiting entry of halide-rich liquids into drains or ground, so labs store waste in dedicated containers for proper treatment. National and university regulations dictate glove choice, benchtop containment, and even disposal contractors. Safety training covers spill clean-up, accidental ingestion, fire, and chemical compatibility. Workers don’t take shortcuts—direct handling of raw powders or solutions without protection earns a reprimand across responsible research labs.
Application Area
Research groups often look past tried-and-true solvents, hunting for ionic liquids like this one to tackle old problems from a new angle. 1-Pentyl-2,3-dimethylimidazolium bromide pops up in synthesis labs focused on catalysis, extraction, and electrochemistry. Battery researchers have tried it in electrolytes, chasing higher ionic conductivity alongside less flammability than conventional organics. In synthetic organic reactions, the liquid matrix helps dissolve polar and nonpolar species at once, letting new pathways open up. Extraction of metals and rare earths, especially those that hate water or standard chemicals, sometimes leans on this salt’s solubility profile. Some green chemistry groups investigate its use as a non-volatile alternative to standard solvents, aiming to cut down on worker exposure and air emissions.
Research & Development
Work does not stop after initial synthesis—every year, university labs publish fresh papers on new ionic liquid modifications, mechanism studies, or property tuning. Chemists track viscosity, dielectric constant, and response to electric fields, searching for connections between small molecular tweaks and bulk properties. Electrochemists plot conductivity curves against temperature, figuring out the limits for next-generation batteries or double-layer capacitors. Computational chemists run molecular dynamics, mapping out hydrogen bonding, aggregation, and solvation shells, nudging theory closer to experiment. Projects sometimes hinge on collaboration, requiring synthetic chemists, analytical wizards, and application specialists to cross-test findings. Intellectual property filings crop up as researchers try to lock down unique uses or novel mixtures, pushing academic results into commercial territory.
Toxicity Research
Reports over the last decade show that not every ionic liquid is as benign as early enthusiasts hoped. 1-Pentyl-2,3-dimethylimidazolium bromide lands somewhere in the middle—not as dangerous as heavy-metal salts or volatile organics, but not completely harmless either. Lab animals exposed to significant doses develop moderate irritation or, at higher levels, organ effects. Biodegradation lags behind household detergents, so environmental buildup can stress aquatic systems. Most research points toward a need for careful use and dedicated waste management, rather than unchecked disposal. A few reports hint at longer-term issues if exposure remains unchecked, so regular monitoring and worker awareness training cannot be skipped. Still, its relatively low vapor pressure reduces acute inhalation hazards in regular lab or small-scale industrial settings.
Future Prospects
Opportunities keep rolling in for ionic liquids that balance cost, performance, and safety. 1-Pentyl-2,3-dimethylimidazolium bromide could see more use in customized solvents, engineered electrolytes, and specialized extractions, especially if regulatory authorities tighten restrictions on classic organics. Sustainable chemistry drives demand for non-volatile, recyclable mixtures, and chemists who tailor structures for biodegradability or easier recovery can reshape the market. Technical obstacles remain in upscaling, purification, and consistent batch production. Increasing collaboration among industries—batteries, green solvents, pharma—offers new targets and broader adoption. Strict adherence to environmental and occupational safety rules will limit hasty adoption but also force the industry to prioritize long-term health and ecological impacts. The compound looks to sit at the edge of growth, waiting for breakthroughs in cheaper synthesis, higher purity, or clear demonstration of gains over familiar rivals.
Understanding Its Role in Modern Chemistry
1-Pentyl-2,3-Dimethylimidazolium Bromide might sound like a mouthful, but this compound finds real traction in labs and industrial setups. Chemists look for substances that boost reactions or open new pathways to manufacture materials safely and efficiently. In my experience, ionic liquids like this one have changed how many industries handle chemical challenges.
Cleaning Up Reactions
One place this compound plays a key role involves the field of green chemistry. Many labs still rely on toxic organic solvents, but ionic liquids come with lower vapor pressures, so they don’t turn into fumes, and often allow for easier recycling. I’ve seen 1-Pentyl-2,3-Dimethylimidazolium Bromide step in as a solvent. Because it dissolves a range of materials, researchers use it to help reactions run faster and, sometimes, with fewer byproducts. Cleaner reactions mean industries spend less chasing down impurities and more time focused on quality output.
Electrochemistry and Energy Storage
Batteries and fuel cells power more than just cars—they keep our phones charged and back up hospitals. Many devices depend on stable electrolytes, which handle ions back and forth without breaking or catching fire. Here, this imidazolium salt stands out. Its structure keeps it stable, even under current and heat. I know battery researchers who use it to reduce degradation in lithium-ion cells. That gives electronics a longer life and helps curb pollution from worn-out devices ending up in landfills. The safety factor carries real appeal, especially as new battery chemistries look to replace old, flammable formulations.
Catalysis: Making Reactions Run Smoother
Many catalysts fail because traditional solvents clash with them or break down under tough conditions. 1-Pentyl-2,3-Dimethylimidazolium Bromide, though, holds up under pressure. It supports transition metal catalysts, letting reactions hit higher yields. In my graduate work, swapping to this ionic liquid meant fewer contaminated samples and cleanup headaches. Industry pushes for efficient processes, and every step saved on quality control means more reliable production and less environmental hassle.
Carbon Capture and Environmental Solutions
Climate change isn’t just a government problem—scientists and engineers tweak processes every year to cut emissions. One piece involves capturing carbon dioxide from exhaust gases. Some ionic liquids have strong absorption capabilities, and research shows that this bromide salt can take up large amounts of CO2 compared to water-based systems. Power plants and cement factories could see a real drop in emissions by switching, as regeneration for reuse comes easier and costs less over time. I think that’s a practical step forward, especially when governments push for greener policy.
Where It Goes Next
The uses for 1-Pentyl-2,3-Dimethylimidazolium Bromide keep growing. More labs adopt it for protein extraction, nanomaterial fabrication, and pharmaceutical synthesis. Safety profiles look stronger than many legacy chemicals. But real impact depends on supply chains, cost, and new regulations. Industry and academic labs should keep sharing results so safer, more efficient alternatives reach real-world processes. With the right support, breakthroughs like these can help shape cleaner, smarter production for years to come.
How This Salt Holds Up
Chemistry labs around the world started looking seriously at imidazolium-based salts a couple decades ago, searching for ionic liquids that promise low volatility and wide liquid ranges. 1-Pentyl-2,3-Dimethylimidazolium bromide sits among those salts that researchers lean on thanks to its tolerance for a range of temperatures and moisture levels. The imidazolium cation doesn’t give in easily to breakdown, which comes as a relief when checking on shelf life and storage safety.
A key reason for this endurance: the pentyl group’s size. A longer alkyl chain on the imidazolium ring brings greater hydrophobic character, making it less likely to react with water in the air. I’ve spoken with colleagues who've left batches of this salt uncapped overnight—sure, not exactly best practice, but nothing catastrophic happened. No odd colors, no unpleasant odors, and, later analysis by NMR or IR, little sign of decomposition.
Real-World Storage Challenges
In practice, storing this compound still requires care. I’ve seen what sunlight can do where someone leaves a bottle under a window: browning, clumping, and sometimes a whiff of decomposition. That tells me photostability is not a strong point here. Keep it in amber glass or a dark cabinet, not out in the open with the coffee mugs.
Temperature swings don’t typically shatter the molecular structure, but extreme heat draws out weaknesses. Labs that keep non-hygroscopic salts in warm warehouses have circled back with minor losses in purity—even with a closed cap, long-term exposure above 40°C invites slow hydrolysis. Not fast enough to go up in smoke, yet not slow enough to ignore for reagents. Bromide ions tend to pick up moisture over time, and if humidity gets too high, the sample feels sticky and less free-flowing.
Watching Out for Chemical Foes
Acids and especially strong bases don’t play well with this salt. One chemist I know accidentally mixed a sample with potassium hydroxide, and the imidazolium ring started falling apart within minutes, showing classic signs of nucleophilic attack at the C2 position. That’s because the positive charge of the ring attracts nucleophiles, and chemically speaking, this can trigger off-flavors and unexpected colors. It’s not explosive, but it’s a waste of perfectly good ionic liquid.
Oxidizers should also stay away. This salt doesn’t catch fire or fume dramatically, but adding strong oxidizing powders or peroxides into the beaker introduces the chance for uncontrolled side reactions. The pentyl chain provides enough carbon that, with the right oxygen-rich compound, burn-off or charring may occur at high temperature.
Tips from the Bench
For routine work, clear labeling and dating of containers keeps things organized and helps track shelf life. Use gloves and safety glasses—no exceptions, since skin exposure to bromide salts can cause mild irritation in sensitive folks. For waste, seal the salt in a plastic bag before tossing it in the designated container, especially if it might mix with acids during disposal.
Some manufacturers add a small drying packet to each bottle—after years in the lab, I keep a stash on my shelf for just this reason. It’s a simple step that goes a long way to keep your salt free-flowing until the last gram.
Why Stability Matters
Stable salts like this open doors for safer handling and more reliable results, whether they end up as supporting electrolytes, solvents, or catalysts. You get less waste. Your reactions keep humming. Your budgets stretch further. That’s not a small bonus in any research environment.
Getting Storage Right Matters More Than We Think
In the lab, quality work depends on solid habits. The last thing anyone wants is to reach for a bottle and find a crusty mess or fumes where a powder used to be. This rings true with compounds like 1-Pentyl-2,3-Dimethylimidazolium Bromide, an ionic liquid with promise in organic synthesis, materials chemistry, and electrochemical applications, but a shelf-life on the line if basics get skipped.
Nobody enjoys lost batches or wasted time, especially when a little planning keeps things clean. Ionic liquids like this one don’t act like table salt or old-school solvents. Moisture can sneak in, sometimes not even from spills, but just from air. Over the years, I’ve seen chemists try shortcuts—reclosing caps loosely, leaving reagents out “just for a minute,” or trusting labels that say “store at room temperature.” The difference between a successful procedure and a failed run sometimes boils down to a habit as simple as closing the lid carefully or tucking reagents away from sunlight.
Controlling Temperature
Most manufacturers recommend a cool, dry environment, often around 20°C or lower. For routine use over weeks to months, a refrigerator between 2°C and 8°C helps. I once thought a regular bench would do, but heat from sunlit windows or nearby hotplates added enough warmth to degrade sensitive compounds. Refrigerators used just for chemicals keep out the risk of food contamination, which can sneak in more problems than most realize.
Moisture and Air Exposure: The Enemy
Even in sealed bottles, moisture from humid air creeps in during rushed handling. Dryboxes save the day here. A simple desiccator with fresh silica gel supports longer-term stability. Whenever the cap unscrews, dry hands matter too.
One year our bench supply developed clumps after just a week of humid summer days. That meant disposal—not worth salvaging—because even if the bottle looked fine, water contamination would ruin the next reaction.
Keep Light at Bay
I learned not to underestimate the power of light. Some imidazolium compounds break down slowly from direct light, especially under bright fluorescent bulbs. Amber bottles help, but even more, I find tucking chemicals away in cabinets beats relying on tinted glass alone.
Choosing the Right Container
Glass works better than plastic for these ionic liquids, as bromide ions can corrode metal lids or react with some plastics over time. Tightly sealed screw caps with PTFE liners make a big difference. Labels—dated and clear—let everyone on the team know the age and who last used a bottle. Nothing slows a day in the lab more than guessing what’s in a mystery vial.
Solutions for Better Practice
Good inventory records flag expiry dates and storage logs. In bigger labs, chemical tracking software helps keep tabs on opening times, last checks, and temperature changes. At home or in smaller groups, adding a log sheet right next to the chemical cabinet does the job. Regular checks, every few months, save reagents and money.
Science works best on predictability. In practice, keeping things simple—clean, cool, dry, and dark—goes further than chasing miracle products. It respects the investment in both the reagent and the results you need. After a few ruined samples and the costs that followed, that habit stuck for good.
Breaking Down Chemical Safety in Everyday Life
Most daily routines don’t involve a tongue-twister like 1-pentyl-2,3-dimethylimidazolium bromide. Just the name alone draws images of fume hoods and gloves stretching up the arms. This chemical belongs to a group called ionic liquids, which often show up in laboratories and research settings. Still, before rolling eyes and shutting this webpage, keep in mind that chemical safety isn’t just for researchers. Every time something gets poured down a drain or touches the skin, the consequences can stick around for a while—sometimes in ways that only surface years later.
Hazard Profile: What We Know
Regulatory agencies keep long records on many industrial chemicals, but reports on this specific imidazolium compound don’t fill as many pages as the ones reserved for lead or mercury. The information isn’t empty—scientific journals track ionic liquids and their effects. Studies flag the potential for these chemicals to irritate skin and eyes. Inhaling powders or vapors can also spark respiratory trouble. Data on animals hint at more harm through longer exposure: organ health can take a hit, and aquatic systems don’t bounce back easily when these compounds wash in.
Experience in the lab means respecting both what’s known and what isn’t spelled out yet. Even with gloves on, accidents can happen. Surface contact with substances similar to this bromide often leads to redness and itching without drawing blood. Spills on a benchtop force quick thinking: chemical splash goggles, plenty of water, and sometimes a call to the safety officer.
Why Clarity Around Hazards Matters
Companies sometimes chase innovation by making tweaks to chemical structures. The imidazolium group is a favorite for fuel cells, batteries, and solvents, and it’s tempting to assume new versions carry fewer risks. That assumption cost humanity dearly with substances like asbestos and PCBs. Living with asthma, I’ve always felt the sting of tiny particles in the lungs. Few scientists shrug off the possibility that novel substances can aggravate underlying health issues or show up in drinking water, even years after the fact. Everyone trusts that “just a little” exposure won’t matter, until low-level contamination piles up.
The practical reality: uncertainty itself poses a hazard. Regulations often lag far behind invention, so workers and communities become test cases. Speed bumps in research slow progress, but they don’t justify sending poorly-understood compounds out into the world. Companies get louder about “green chemistry,” but often, marketing doesn’t keep up with deep safety checks.
Building a Safer Future
Nobody can roll back the chemical advances of the last century, but more thoughtful risk assessments can shrink future regrets. For any compound like 1-pentyl-2,3-dimethylimidazolium bromide, demand clear labels, easy-to-read safety data sheets, and honest reporting of accidents. Full transparency beats scientific jargon every time—it puts the power back in the hands of those working near these chemicals every day.
Substituting less hazardous materials, installing better ventilation, and running routine health screenings make a stronger case for innovation than any quarterly profit report. Public research funding should set aside more support for long-term toxicity studies, rather than just leaving small-scale hazards for someone else to clean up. As demand rises for cleaner indoor air and safe water, leaving safety to chance isn’t an option. Anyone involved in new chemical development ought to remember: the real experiment starts when the product leaves the lab.
Why Purity Really Matters
Buying chemicals for research or production isn’t like buying flour for cookies. Purity acts as both a guarantee and a risk, depending on which side it lands. With 1-Pentyl-2,3-Dimethylimidazolium Bromide, a salt used in everything from pharmaceuticals to energy storage experiments, the level of impurities determines how reliable your results turn out. One impurity, and that experiment you’ve spent weeks on suddenly spins out of control.
When I worked with ionic liquids, there were times impurities caused color changes, odd smells, and totally threw off every yield calculation. It’s easy to scoff at “99% pure” labels until something fails, and the blame can’t be traced anywhere else. Lost time, wasted money—and worse, doubt in every data point you now have to check again because of a tainted batch.
What Chemists Need to Know About Purity Levels
Chemicals like 1-Pentyl-2,3-Dimethylimidazolium Bromide typically get sold at purities above 97% for lab use. Analytical work, especially NMR spectroscopy or battery testing, usually needs at least 98% or 99%. Impurities like water, leftover starting materials, or metals can change how the salt behaves in a reaction or an electrochemical cell—sometimes even in subtle, invisible ways.
Purity isn’t just a number. Labs often push for certificates of analysis with specific impurity breakdowns. One company might promise 99% purity by NMR, but a single percent of chloride instead of bromide in the mix can shift the entire chemistry. Never just read the purity line—demand a full report.
How Purity Gets Tested in the Real World
Quality control labs rely on several techniques to check for purity. NMR and IR spectroscopy help spot organic impurities, while elemental analysis and ion chromatography flag metal traces or unwanted anions. From my own experience, a lot of “white powders” look identical on the lab bench but have different melting points or hygroscopic behavior because of hidden contaminants. Nobody wants a salt that starts absorbing water just because of a tenth of a percent of something unexpected.
Working with junior chemists, I’ve seen how easy it is to trust a label and skip verification. Training new researchers to check melting points and run NMR as soon as a product arrives cuts down on big mistakes. That sense of trust in your materials can only be built through a habit of double-checking.
Building Better Practices for Purity
Chemical suppliers who care about reputation don’t hide behind vague numbers. It helps to choose vendors who offer transparency, regular batch testing, and responsive technical support. If a supplier can’t or won’t send detailed impurity analyses, move on. Researchers deserve to know if trace solvents, extra halides, or leftover starting materials linger.
Academic labs and startups both lose precious time and money from materials that secretly fall short of “pure.” Pushing for detailed testing—before scaling up any process—saves on troubleshooting and embarrassment. Connecting with trusted vendors and sharing experiences among colleagues sharpens everyone’s instinct for spotting inconsistencies.
Moving Ahead with Confidence
Solid research relies on knowing the quality of every reagent. For something as sensitive as 1-Pentyl-2,3-Dimethylimidazolium Bromide, don’t just trust the number on the catalog. A little skepticism and a habit of checking can turn headaches into confidence, leaving more room for real breakthroughs.