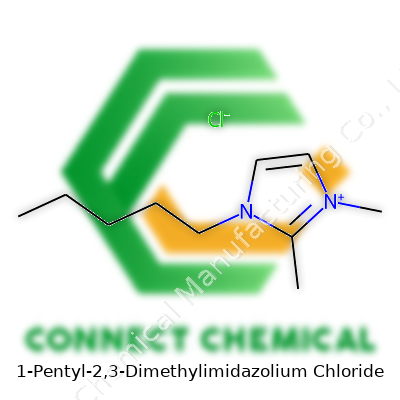
1-Pentyl-2,3-Dimethylimidazolium Chloride: Commentary on Development, Application, and Safety
Historical Development
Once chemists realized imidazolium-based ionic liquids carried interesting properties for green chemistry, new variants kept popping up in research labs. Years ago, the core structure, a five-membered ring with two nitrogens, set the base. Substituting different groups onto this skeleton gave birth to unique salts including 1-pentyl-2,3-dimethylimidazolium chloride. Knowledge grew out of necessity—people working in solvent design for metal extraction or synthesis wanted alternatives that could handle extreme temperatures and cut volatility. In the two decades since, academic literature documented the shift from old-school solvents to these designer liquids, pinpointing gains in solubility, selectivity, and thermal stability.
Product Overview
1-Pentyl-2,3-dimethylimidazolium chloride emerged as a specialty ionic liquid, distinguishing itself by mixing the right side-chain length with manageable melting points. Chemists refer to its shorthand as [C5mmim]Cl or similar tags. Interest sharpened as it tackled roles in organic synthesis, catalysis support, and advanced materials processing. Unlike some salts, it dissolves a broad range of organic and metal species, which hints at versatility for extractive or synthetic applications. Laboratories pursuing custom solvent systems or a safer alternative to conventional halogenated solvents turned to this molecule for its blend of reactivity control and lower vapor emissions.
Physical and Chemical Properties
This compound pours as a pale, sometimes viscous liquid at room temperature, with a typical faint color—sometimes yellowish or colorless—depending on purity. Molecular formula reads C10H19N2Cl, weighing in at about 218.72 g/mol. The chloride anion gives it solid ionic character, stabilizing the salt under heat. Its melting point stays just below 45°C in most batches, though real-world values can vary with trace impurities. Density numbers hover between 1.0 and 1.2 g/cm3. Thermal stability stretches well above 200°C, which is crucial for operations involving sustained heating. Water solubility appears moderate, enough for use in two-phase systems, but it gels quickly with more hydrophobic solvents. Its cationic imidazolium core encourages hydrogen bonding and π-π stacking, which tailors solvation properties in multi-component reactions.
Technical Specifications and Labeling
Producers of 1-pentyl-2,3-dimethylimidazolium chloride bottle it as a specialty chemical, usually at 98% or higher purity. Certificate of analysis paperwork often lists residual water content, halide content, and UV-Vis spectra to confirm identity. Customers expect lot-specific MSDS labeling, with hazard pictograms that point to potential skin or eye irritation. Storage advice on bottles emphasizes keeping the material away from humidity or air, as prolonged exposure could trigger hydrolysis and breakdown products. Beakers and glassware pick up a slick residue because of the salt’s slight oiliness. Producers highlight the need to avoid copper or brass tools, since the chloride ion encourages corrosion—this reality keeps equipment choices honest.
Preparation Method
Making this ionic liquid typically begins in a flask with N,N-dimethylimidazole and 1-pentyl chloride loaded in equimolar amounts. Chemists heat the mixture gently under inert gas, encouraging the substitution reaction that attaches the pentyl group to the imidazolium ring. Once the salt forms, the batch turns thick and sticky. Purification calls for repeated washing—sometimes with ethyl acetate or toluene—to strip out unreacted starting material. Drying under vacuum strips off volatile leftovers, leaving behind the pure product. Scale-up produces challenges; batch-to-batch consistency hinges on careful ratio control, solvent removal, and avoiding side reactions that might yield methylated byproducts or overalkylation.
Chemical Reactions and Modifications
1-pentyl-2,3-dimethylimidazolium chloride reacts predictably, sticking to the typical imidazolium salt profile. It hands off the chloride for anion exchange, letting labs swap in PF6-, BF4-, or other counterions to craft tailor-made ionic liquids. The imidazolium center stabilizes transition states in nucleophilic substitutions, sometimes catalyzing reactions or acting as a phase-transfer agent in tricky syntheses. In strong base, the cation ring shows enough stability to resist ring opening, though under more strenuous conditions, such as high heat with strong nucleophiles, breakdown can occur. Chemists attempting to graft this molecule onto polymers or anchor it to silica use the chloride as a handle for further functionalization.
Synonyms and Product Names
In chemical catalogs, the compound takes several aliases, including [C5mmim]Cl, 1-pentyl-2,3-dimethylimidazolium chloride, and sometimes Pentylmethylimidazolium chloride. Some sources tag it with registry numbers for tracking, though commercial batches usually stick to the common name or shorthand. Naming conventions sometimes shuffle the order of methyl and pentyl substituents, which means double-checking molecular structures stays necessary to avoid cross-ordering or misinterpretation.
Safety and Operational Standards
Lab safety teams treat imidazolium ionic liquids with respect. This chloride salt can cause irritation if it lands on skin or in eyes and needs clean bench handling. Gloves and goggles do the job. Ventilation must handle organic vapors from wash solvents more than the salt itself, since it barely volatilizes. Spills clean up with detergent and water, though any contact with copper surfaces leaves a risk for slow pitting corrosion. Chemical waste collection guidelines classify it as a hazardous material downstream due to persistent organic content. Procedures insist on dry storage and keeping lids tightly closed. For folks working with larger volumes, spill containment kits and training in first response techniques form a reliable safety net.
Application Area
This ionic liquid found its place in experimental organic synthesis as a solvent for transition metal catalysis, stubborn coupling reactions, and biphasic extractions. Electrochemistry teams use it for ionic conductivity studies, since the chloride helps shuttle electrons in electrolytic cells. Battery research pointed toward this compound for its temperature resilience and resistance to solvent evaporation, making it a fit as an electrolyte additive. Pharmaceutical researchers dipped into its solvating properties to test routes for purifying new drug substances. Down the line, extraction experts worked the salt into two-phase systems for separating metals or rare earth elements from ores, which simplified downstream recovery and recycling of both solvent and product.
Research and Development
Research groups invested time comparing 1-pentyl-2,3-dimethylimidazolium chloride’s effectiveness to related ionic liquids. Structure-property relationships receive lots of focus—modest tweaks in side chains or counterion choice drive big changes in solubility, chemical stability, or reaction yield. Teams documented improved selectivity in catalytic oxidations, fewer side products in alkylations, and tighter control of particle size in nanoparticle synthesis. Publications mapped out greener protocols by replacing VOC-heavy solvents with this salt, supporting industry’s push for cleaner processes and reduced emissions.
Toxicity Research
Toxicologists stayed cautious about environmental impact and human exposure. Early animal studies suggested acute toxicity thresholds above everyday handling levels, but chronic bioaccumulation remained unclear. Ionic liquids frequently resist rapid breakdown; their persistence in waterways spurred debate about long-term ecosystem risks. Most research flagged imidazolium salts as mild irritants, not outright poisons, but noted that mixing with heavy metals or certain organics could elevate hazards. Wastewater analysis uncovered trace levels after manufacturing runs, prompting regulatory calls for rigorous monitoring. Labs working on new versions experimented with biodegradable side chains, but full biocompatibility seemed a few years away.
Future Prospects
Development continues as industries seek out solvents and electrolytes that mix chemical stability with environmental safety. If regulatory agencies agree on clear benchmarks for persistence, toxicity, and recyclability, 1-pentyl-2,3-dimethylimidazolium chloride stands to stay relevant for specialty synthesis and advanced battery research. Cutting down residual chloride in recovered waste streams could quiet environmental criticism. Chemists hunt for derivatives that work even in bio-based separation or pharmaceutical purification, where mildness and low residue prove crucial. With focus turning to circular economy principles, the challenge lies in balancing performance against impact—recycling, safer manufacturing, and full-life-cycle management chart the way forward.
What Sets 1-Pentyl-2,3-Dimethylimidazolium Chloride Apart?
1-Pentyl-2,3-Dimethylimidazolium Chloride doesn't show up on the average shopping list, but in scientific and technical circles, it's hard to ignore. This compound falls into the category of ionic liquids, those salts that melt below 100°C and offer unique features compared to water or oil. They can dissolve all sorts of materials and stay stable under tough conditions. When I first came across it in a research setting, the promise was clear: a solvent that stays put and opens new doors in chemistry and engineering.
Lifting Barriers in Green Chemistry
Green chemistry gets a lot of lip service, but progress happens on the backs of specific materials like this one. 1-Pentyl-2,3-Dimethylimidazolium Chloride steps up because it doesn’t evaporate easily and isn’t flammable, which cuts risk in labs and factories. I’ve seen teams lean into ionic liquids to replace traditional organic solvents that carry big environmental baggage. In the field, chemists use it to run reactions that need high temperatures or air-sensitive setups, without worrying about dangerous fumes.
Dissolving and Separating Materials
If you spend time around cleaning up industrial waste or extracting pure ingredients from plants, ionic liquids sit at the top of the wish list. This compound helps break up stubborn materials like cellulose, which forms the backbone of wood and cotton. Once dissolved, these building blocks aren't hard to shape, study, or transform. I've seen 1-Pentyl-2,3-Dimethylimidazolium Chloride used in biomass conversion, where people look for cleaner ways to create biofuels and new plastics. The efficiency comes from the compound's knack for breaking down tough, tangled fibers without creating a hazardous mess.
Powering Up New Batteries
Energy storage keeps grabbing headlines, and the search for safer, longer-lasting batteries never ends. Some researchers have plugged this ionic liquid into battery prototypes. The big win? Electrolytes based on this compound resist catching fire and break down less quickly during charge cycles. Lithium-ion batteries get safer and snag a longer lifespan. From my experience, the push for battery tech always meets resistance from lithium fires and strict safety rules. Testing ionic liquids has tempered these risks, making ambitious battery projects less of a gamble.
Catalyst for Smarter Chemical Factories
Chemical companies dream of efficient, low-waste factories. This compound often turns up as a medium for catalytic reactions. It tunes the environment for chemical reactions so that more product comes out with less waste and fewer spills. Colleagues in process engineering have used this approach to shave costs and keep operations running cleaner. For instance, hydrogenation and alkylation reactions pick up speed and yield using ionic liquids like this one, since they dissolve both polar and non-polar chemicals.
Pushing Toward Safer Industry
With traditional solvents, I've watched accidents happen and clean-up costs soar. Regulatory headaches aren’t just theory; they stall projects and eat budgets. 1-Pentyl-2,3-Dimethylimidazolium Chloride draws attention not only for what it does but what it avoids. Fewer volatile organic compounds leave the shop, and that builds a safer workspace. As adoption grows, the focus lands on recycling these ionic liquids—most can be cleaned and reused, dodging the single-use problem that plagues many chemicals.
What’s Next?
Solvents have shaped modern industry. Safer, more versatile options like this one could help tackle major environmental and engineering challenges, provided enough investment flows toward improving their production and recycling. Labs and companies that want fewer risks and greener credentials owe these ionic liquids a closer look.
Why Chemical Purity Isn’t Just a Number
Purity gets more attention than most things in the laboratory world—and for good reason. People often ask about the chemical purity of compounds like 1-Pentyl-2,3-Dimethylimidazolium Chloride before they turn to any experiment or production run. Digging through safety data sheets, one usually stumbles across values like “>98%” or “99% pure.” Maybe those numbers should feel reassuring, but anyone who’s run experiments with ionic liquids or specialty chemicals knows the whole story doesn’t sit in a single percentage.
Understanding Why Purity Matters—From Real Life to the Lab
I remember the first time I tried to synthesize a catalyst using an imidazolium salt. Everything went as the textbook promised, but the reaction yield stayed stubbornly low. Chasing down the problem, I learned the imidazolium salt I ordered clocked in at 97% by weight, but nobody told me about those minor impurities. In sensitive chemistry, a difference of even 1% can make or break a process. Those side products often change color, boost corrosion, or add unexpected volatility.
For 1-Pentyl-2,3-Dimethylimidazolium Chloride, the reported chemical purity on spec sheets from most major suppliers tends to hover around 98-99%. But tucked behind that figure, manufacturers often blend in trace solvents, halide remnants, and moisture. These ghosts sometimes slip under the radar, especially in cost-driven or bulk chemical markets. If you’re in battery research, catalysis, or pharma—small impurities have serious consequences. Electrochemistry, for example, punishes stray ions and water content because they alter conductivity. Drug development needs every molecule counted, and random side products threaten safety.
What Real Testing Looks Like
Chemical analysis should do more than a single measurement. Labs use techniques like NMR, HPLC, and mass spectrometry to show if anything unusual appears in a batch. Some of the most respected suppliers give a full breakdown—including water by Karl Fischer titration, halide levels, and specific organic impurities. I’ve seen batches pass all the standard checks and still fail for unknown UV-active contaminants visible only at low concentrations, so extra diligence pays off.
How to Improve the Situation
If chemical purity leaves you second-guessing your results, don’t just trust the label. Always ask for a Certificate of Analysis. Push for third-party confirmation if you’re working in sensitive applications. Consider using in-house NMR or HPLC to double-check. If vendors can’t give you a recent analysis with impurity profiles, look elsewhere. Building long-term relationships with suppliers who keep detailed records matters just as much as any fancy purification step.
Better transparency could help. An industry-wide shift toward batch-specific certificates, more detailed impurity lists, and full disclosure about residual solvents or side products would give chemists—including students and entrepreneurs—the confidence they deserve. Until then, double-check the paperwork, question unexplained losses, and don’t let a few numbers on a spec sheet lull you into false confidence. The science you do with 1-Pentyl-2,3-Dimethylimidazolium Chloride stands or falls on what’s really in that bottle.
Why Storage Really Matters for Lab Chemicals
The stability of ionic liquids like 1-Pentyl-2,3-Dimethylimidazolium Chloride matters because even a small change can throw off a whole research project. I remember shelving a bottle of an imidazolium salt, thinking the fridge would always be enough, but a leaking cap and humid air almost ruined a month’s work. The risks go beyond wasted effort. Degrading chemicals can contaminate samples, create safety hazards, and drive up costs if they need to be replaced.
The Critical Role of Dry Conditions
This compound draws moisture from the air. Leaving the bottle open on a bench tempts fate. Even a tightly screwed cap won’t always save things unless the storage environment stays dry. I prefer placing such bottles in a desiccator. Desiccators with silica gel or molecular sieves pull out moisture and keep the salt dry — which means the melting point and chemical behavior stay consistent each time.
Keep It Cool and Stay Consistent
Heat speeds up decomposition in many ionic liquids. A cool room or a dedicated chemical fridge, away from temperature spikes, helps protect the chemical’s structure. Room temperature sometimes works, but sticking to below 25°C reduces surprises. In my experience, temperature swings during a hot summer badly affected the quality of similar salts. It pays off to check the thermometer on storage appliances regularly instead of just assuming things stay cool because the light is on.
Avoiding Light and Oxidizing Agents
Many imidazolium salts break down under strong light. Fluorescent bulbs or sunlight both create trouble. Store the bottle in a dark cabinet or use amber containers if the room gets plenty of daylight. Avoid sharing a shelf with oxidizers. Chemicals like peroxides might not seem immediately reactive with this salt, but leaks or accidental spills can start messy reactions. In shared labs, a bit of tape to label shelves works better than relying on memory.
Sealing and Labeling: More Than Just Rules
Tightly sealed containers block air, dust, and contaminants. I replace the cap quickly after use, and avoid transferring salts to multiple bottles. Every move raises the risk of contamination. Clear labeling, including the compound’s name and open date, sounds basic but saves confusion, especially for new lab members. In one project, an unlabeled bottle led to a mix-up that forced us to repeat an entire experiment. It’s a small detail, but it stops big mistakes.
Routine Checks and Getting Everyone on the Same Page
Regular spot checks go a long way: look for clumping, color changes, or strange smells. If anything seems off, I set the bottle aside until I can double-check purity. Training anyone who works with chemicals beats relying on written procedures alone. Repeating best practices helps ensure routine storage becomes habit, saving a lot of hassle over the long term.
Takeaway: Simplicity Beats Complexity
Protecting 1-Pentyl-2,3-Dimethylimidazolium Chloride comes down to a dry, cool, dark storage spot plus airtight bottles and clear labels. These simple steps keep costly, sensitive chemicals in top condition. Over time, I’ve learned that good storage sets the foundation for every safe, successful experiment.
Real Concerns in the Lab
1-Pentyl-2,3-dimethylimidazolium chloride shows up often in conversations about ionic liquids and modern chemistry labs. I’ve handled my share of specialty chemicals and always keep safety at the front of my mind. Experience has shown me that substances with long, unfamiliar names often need a closer look before they end up on a bench or in a beaker.
Why Pay Attention?
This compound belongs to the family of imidazolium salts, which cropped up in green chemistry circles because they serve as ionic liquids for solvents and catalysts. Some folks assume that “ionic liquid” translates to “safe” just because these don’t evaporate like common solvents. Sadly, that’s not always the story. The way a chemical acts on skin, lungs, and the environment can surprise anyone, even people who fancy themselves seasoned chemists.
I’ve known people who became lackadaisical with new solvents, almost like things that don’t fire up instantly or stink can’t hurt anyone. Eventually, they learn the hard way. Some ionic liquids damage cells, irritate skin, or – after weeks of regular use – show up as mysterious headaches or rashes. The fact is, lab accidents rarely happen to those who read the data, but instead to those who rely on rumors.
Digging into the Facts
No household-name government agency hands out a scorecard for every ionic liquid. Instead, the Material Safety Data Sheet (MSDS) offers the most practical guide. For 1-pentyl-2,3-dimethylimidazolium chloride, most sheets flag possible irritation to skin and eyes, and warn not to breathe in the dust. Some studies connect imidazolium compounds with cell damage in certain organisms and moderate toxicity in aquatic settings. Chronic exposure, accidental spills, and unfiltered air can lead to health concerns.
Green chemistry has taken big steps, but “less hazardous” doesn’t equal “harmless.” A chemical that sticks around in the environment, or travels down the drain, can become toxic for fish or other wildlife. Restrictions sometimes lag far behind emerging risks. Real harm can show up unnoticed until someone asks the right question.
What Does Safe Handling Look Like?
Gloves are non-negotiable. Eye protection feels like a second skin in my lab, especially with chemicals like this on the table. Even if accidents don’t happen every week, chemical burns sting and sideline researchers with one slip. Fume hoods make sense, since nobody in their right mind wants to gamble with inhalation risk. Ventilation keeps repeat exposure low and air fresh for the next person. Storing any imidazolium salt in sealed, labeled bottles cuts confusion and keeps cross-contamination low. I label everything twice, because mistakes creep in when everyone is tired or in a hurry.
Main surfaces deserve regular wipes with a detergent, and never leave droppers or spatulas lying around. Pouring unused chemicals back into original containers creates headaches—contamination wrecks entire batches. Sharps disposal and spill kits belong close by. Training isn’t paperwork; watching the old hands teaches more than a PowerPoint ever could. It all comes down to a habit of respect. One wrong move changes a good day into an accident report.
Problems and Paths Forward
One of the hardest problems comes from unpredictability. Universities and companies benefit from better access to up-to-date toxicity data, and personal stories sometimes hold more power than written guidelines. Beyond paperwork, open discussions in research groups drive real improvements. Safety officers need face-time with chemical users to figure out who cuts corners and who keeps the place running smooth. Inventors of new ionic liquids could share more about long-term hazards, not just the economic upsides.
The spark for better habits doesn’t come from reading a single sheet. It comes from sharing lessons learned, and building a culture where people speak up the minute a risk pops up. Chemicals like 1-pentyl-2,3-dimethylimidazolium chloride bring valuable tools to research, but safety begins well before the first test tube fills up.
Understanding the Stakes
Any talk about 1-Pentyl-2,3-Dimethylimidazolium Chloride in industry circles sparks the same big question: is sourcing this ionic liquid in large batches practical, not just in theory, but in day-to-day business? In manufacturing, reliability isn’t a luxury—it’s vital for survival. What I’ve seen across the chemical sector is that any specialty compound, especially those with tricky organic cation structures, brings unique demands that test supply chains and supplier trust.
Demand and Production Bottlenecks
Companies interested in this particular imidazolium salt generally want it for a collection of high-value purposes like advanced batteries, customized solvents, or research in green chemistry. Lab batches are straightforward—order a few grams, pay a premium, get a neat glass bottle on your loading dock. Scale up to hundreds of kilos, though, and the game changes. Suddenly, you rely on manufacturers running reactors round the clock, sourcing precursors that haven’t dried up due to someone’s export policy, and keeping impurities below strict thresholds. A single hiccup in the supply chain—think of a fire at a plant producing an upstream intermediate, or a geopolitical tussle affecting international shipping—brings production to a screeching halt, taking down timelines and budgets with it.
Quality Counts, Not Just Volume
I’ve seen engineers spend as much energy hounding suppliers about consistency as they do on price. For imidazolium salts, a few parts per million of residual solvent or water content can scramble performance. Bulk volume makes it harder: larger vessels make blending and drying more complex, leading to subtle differences batch to batch. Here, supplier transparency with batch analytics matter more than glossy certificates. Fact is, some labs talk a big game about bulk production but only repackage from smaller runs, hiking costs and risking cross-contamination.
Regulatory Hurdles
Chemicals destined for industrial processing and research move through a minefield of regulation. 1-Pentyl-2,3-Dimethylimidazolium Chloride isn’t a household name, but it still gets noticed. In some places, transportation gets flagged due to its classification, and the paperwork for bulk shipments chews up time. Safety data sheets must be accurate, and customers—in my experience—will grill suppliers for toxicology and environmental impact data, especially if waste handling is expensive or uncertain.
Who Delivers the Goods?
A handful of chemical producers with solid track records take on custom ionic liquids. They’ve invested in big reactors, and they share production data when asked. Outfits based in Europe and Asia usually come up in conversations, as their plants have both ISO certifications and enough capacity to ease corporate anxieties. Middlemen pop up promising cheap deals—but large buyers get burned on quality or delivery times more often than they’d like to admit.
Practical Solutions
Companies across the chemical sector see value in strong partnerships with suppliers, not in chasing spot prices every quarter. I’ve watched teams succeed by locking in transparent contracts, sharing forecasts months ahead, and even paying small premiums for scheduled, reliable deliveries. Some industrial buyers have consolidated orders with similar firms to reach volumes that suppliers take seriously. Technical staff spend more effort on-site audits, inspecting not only the big reactors but the drum-cleaning and labeling lines. It pays off—fewer surprises, fewer missed shipments, and far less stress on project managers trying to keep lines moving and bosses off their backs.
Looking Ahead
Demand for advanced ionic liquids isn’t going away, and companies willing to invest in deep supplier relationships and proactive management usually find solutions. The real test isn’t whether someone can make a few kilos. It’s whether clean, reliable tons show up on time, every time, without drama. That’s what real industrial buyers watch for, because a missed shipment costs more than just a few days on the calendar.