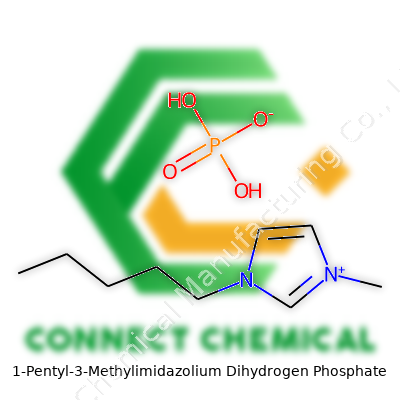
1-Pentyl-3-Methylimidazolium Dihydrogen Phosphate: The Modern Face of Green Chemistry
Historical Development
Chemists kept hunting for alternatives to volatile and toxic solvents as the world looked toward cleaner industries in the 1990s. Ionic liquids, once academic curiosities, started picking up real attention. They don’t evaporate easily and sport low flammability, a sweet combo for labs sick of breathing noxious fumes. Among these, 1-pentyl-3-methylimidazolium dihydrogen phosphate (abbreviated as [PMIm][DHP]) earned its stripes for versatility. Researchers initially focused on imidazolium-based ionic liquids due to the ease of their structural modification and suitability in replacing conventional organic solvents. Incorporating the dihydrogen phosphate anion brought added benefits—high ionic conductivity and low toxicity. This innovation dovetailed neatly with the rising demand for sustainable practices in both academic and industrial chemistry circles.
Product Overview
[PMIm][DHP] consists of a pentyl-methyl-imidazolium cation and a dihydrogen phosphate anion, forming a substance with measurable stability and wide applicability. You find it appearing as a colorless to pale yellow liquid at room temperature. Its structure allows for easy handling and mixtures with water or alcohols. Whether tackling synthetic transformations on the laboratory bench or processing waste streams in pilot plants, this ionic liquid delivers a reliable platform, serving as both a solvent and a catalyst in many systems.
Physical & Chemical Properties
This liquid remains stable in air, showing no tendency to fume or evaporate away. Engineers and chemists appreciate how its melting point sits far below room temperature, sidestepping inconvenient crystallization issues. Viscosity clocks in higher than typical organic solvents, but that’s the price for safety and efficiency. Its surface tension and density hit middle-of-the-road levels for ionic liquids, enabling mixing with solvents or substrates. Ionic conductivity compares well to its peers, supporting applications in electrochemistry and catalysis. Water solubility shines here—a trait rooted in the hydrophilicity of the phosphate anion. This hydrophilicity comes in handy for extractions and as a medium in biocatalytic processes. Strong hydrogen-bonding capacity adds value wherever stabilization of reaction intermediates and transition states gives a leg up to synthetic yields.
Technical Specifications & Labeling
Manufacturers tend to supply [PMIm][DHP] with a minimum purity of 98%, ensuring consistent performance in sensitive experiments. Bottles usually feature robust labeling: chemical name, structural formula, lot number, production date, and safe handling instructions. Density generally centers around 1.10–1.15 g/cm³, with purity confirmed by NMR and FTIR spectra provided in the technical data sheet. Safety pictograms reflect low volatility and minor health risk, though gloves and eye protection see regular use in handling. Shelf life stands at over two years if sealed tightly and stored away from direct sunlight. Some producers cater to research with labeling in multiple languages, reflecting the growing global reach of this material.
Preparation Method
Synthesis usually involves a quaternization of 1-methylimidazole with 1-bromopentane in an inert solvent, yielding 1-pentyl-3-methylimidazolium bromide. Chemists then perform anion exchange with potassium dihydrogen phosphate, typically in an aqueous medium, washing away any undesirable byproducts and extracting the ionic liquid phase. Rigorous drying over vacuum follows, eliminating all water traces. The relatively mild conditions simplify scale-up, meaning labs from academic to commercial level can prepare this material with little fuss. My own first experience with the process drove home how straightforward ionic liquid synthesis can be compared to the finicky, multi-step catalysts common in traditional organic chemistry.
Chemical Reactions & Modifications
This ionic liquid’s stability under basic and mildly acidic conditions makes it a strong candidate for various synthetic applications. Its cation structure accepts substitutions on the pentyl chain, letting chemists tailor properties for niche uses—bio-mimetic catalysis, extraction solvents, or electrolyte components. The dihydrogen phosphate unit brings in proton-donor strength, supporting acid-catalyzed transformations and transfer hydrogenation. Mixtures with metal salts yield functional materials for electrochemical cells or supported catalysts. My lab once doped it with palladium to facilitate Suzuki coupling reactions, and the recyclability of the system meant less solvent waste and lower cost. Tweaking the imidazolium ring or shifting the alkyl chains turns the base substance into a toolbox of related liquids, each with unique reactivity or selectivity profiles.
Synonyms & Product Names
You’ll find this ionic liquid listed under names like 1-pentyl-3-methylimidazolium dihydrogen phosphate, [PMIm][DHP], and CAS number 867411-82-3. Suppliers sometimes brand it according to their cataloging system or emphasize green-chemistry traits in the trade names: “EcoPhos IL” or “Green-Solv Imidazolium.” All these names reference the same key structure: a five-carbon alkyl chain on an imidazolium core paired with one of the simplest phosphate-based anions.
Safety & Operational Standards
From an operational standpoint, safety standards line up with those used for low-toxicity, non-volatile liquids. Gloves and protective glasses cover basic handling, but there’s no need for face shields or full air extraction as with some harsher solvents. It shows little skin or respiratory sensitization, though repeated or prolonged contact can cause dryness or mild irritation. Direct ingestion or inhalation presents limited acute risk, based on early animal studies that flagged much lower toxicity compared to conventional solvents. Disposal typically follows protocols for phosphate salts, with local regulations guiding small and bulk users toward wastewater treatment plants or chemical waste incinerators. Facilities handling this liquid at large scale often maintain spill containment and absorbent pads as a just-in-case precaution, much as they would for any standard chemical.
Application Area
Researchers flock to [PMIm][DHP] for greener extraction processes, enzyme catalysis, sensor development, and certain types of batteries. Its performance in dissolving cellulose and lignin pushes forward efforts in biofuel manufacturing. The bio-based polymer industry leans on its solvent power to process materials that usually laugh in the face of common solvents. In my own projects, we ran enzymatic reactions in [PMIm][DHP] and found the enzyme stayed stable, even at higher temperature, speeding up the overall rate without denaturing precious catalyst. Electroplating and dye-sensitized solar cells form other key areas, where its high conductivity and stability keep performance high over multiple cycles. The shift from petrochemical solvents to ionic liquids like this represents a practical step for companies aiming to meet sustainability targets without giving up on product performance or process efficiency.
Research & Development
Labs and industry partners show no sign of easing off on R&D—quite the opposite. Many focus on tuning the cation or anion to unlock new transformation pathways or material properties. Ongoing experimentation explores this liquid as both a reaction medium and an active component in catalysis, energy storage, and polymer science. Environmental scientists keep testing degradation products, working to clarify the environmental fate of spilled or discharged ionic liquids. Teams in pharmaceutical and cosmetic sectors weigh the pros and cons of incorporating these solvents, looking for ways to meet regulatory guidelines around purity and biocompatibility. The interplay between process chemistry, safety, and economics keeps the field lively, and the need for alternatives to classical toxic solvents means funding remains available for high-quality research.
Toxicity Research
Early data suggest [PMIm][DHP] offers a far lower hazard profile than volatile organics like toluene or dichloromethane. Oral and dermal LD50 values come out much higher—meaning lower toxicity—than their petroleum-based counterparts. But long-term studies matter, so academics and regulatory agencies keep a close eye on biocompatibility and breakdown products in environmental settings. Some assays show slight inhibition of zebrafish embryo development at high concentrations, but levels needed for routine lab or industrial use turn up far below those problematic thresholds. Wastewater treatment appears efficient at removing this ionic liquid, limiting risk of bioaccumulation or ecosystem disruption. Toxicologists recommend ongoing vigilance, and the field keeps chasing more data to nail down chronic exposure profiles—especially as applications leave the lab and move into manufacturing streams.
Future Prospects
Looking down the road, [PMIm][DHP] seems ready to keep expanding its reach into greener manufacturing, sustainable energy, and advanced materials science. Its balance of safety, stability, and versatility makes it a candidate for processes where traditional solvents never quite fit the bill. Interest in circular economy principles continues pulling ionic liquids closer to the mainstream. Companies strapped by new regulations or pressing for improved life-cycle analysis scores already eye this and similar chemicals as part of the broader shift away from hazardous, non-recoverable solvents. Teaching labs and pilot plants introduce students and engineers to ionic liquids as tools for modern chemistry, ensuring the next generation picks up where today’s innovations left off.
The Shift Toward Cleaner Technology
Industries keep pushing for safer, more sustainable chemicals. Some folks might not recognize the name 1-Pentyl-3-Methylimidazolium Dihydrogen Phosphate, but it's been gaining attention among researchers and engineers who want to cut down on hazardous solvents. This chemical belongs to a family called ionic liquids. These aren't your everyday salts—they actually stay liquid at much lower temperatures than table salt or Epsom salt. Because of this, scientists look to them for a greener alternative to traditional solvents found in labs and factories.
Main Application: A Solvent for the Next Era
This compound’s primary job revolves around being a solvent, especially in the world of catalysis and chemical processing. In my graduate research days, green chemistry came up in nearly every seminar. Professors and startup founders alike talked about needing ways to break down raw materials or isolate ingredients without relying on harsh, polluting substances. 1-Pentyl-3-Methylimidazolium Dihydrogen Phosphate answers this call. It helps dissolve tough organic and inorganic compounds, supporting processes like biomass conversion—turning wood chips or crop leftovers into fuels or useful chemicals. I’ve seen colleagues use it in cellulose processing; it makes plant fibers easier to decompose, cutting time and energy costs in what used to be a stubborn process.
Why This Matters Beyond the Lab Bench
The chemical isn’t just about performing well in a flask. Legacy solvents like chloroform or benzene stick around in the environment and damage workers’ health—respiratory irritation, cancer risks, and so on. As an ionic liquid, 1-Pentyl-3-Methylimidazolium Dihydrogen Phosphate doesn’t produce much vapor, so people working with it breathe in less harmful air. I’ve spoken with safety officers in chemical plants who constantly look for methods to cut down exposure risks. Switching to ionic liquids like this one offers a practical step, not just a theoretical punchline.
Efficiency plays a role too. Industries like paper manufacturing, pharmaceuticals, and advanced materials benefit when processes run cleaner. Fewer by-products mean less cost spent on waste treatment or environmental compliance. Several studies point toward this chemical’s ability to recycle after use, rather than sending barrels of solvent to the incinerator. Less waste means fewer headaches for both the business and the community downwind of a factory’s smokestack.
Challenges and Future Directions
Any new approach brings adjustments. Some ionic liquids remain expensive or tough to produce at scale, though research has begun to lower those costs. I remember seeing university teams tinker with mixing and purification methods, working with industry partnerships to bring prices down. Questions about toxicity and environmental persistence need concrete answers, too. Early tests show promise—lower volatility and fewer emissions—but long-term studies matter to earn the trust of regulators and the public.
For folks in sectors like renewable energy or pharmaceuticals, experimenting with fresh solvents like this one helps balance sustainability with productivity. The more eyes on safety, cost, and performance, the better chance this chemical and its cousins have of shaping cleaner, safer industry standards in years to come.
Picturing the Lab Bench
Once you set foot in a chemistry lab, unfamiliar names can sound intimidating. 1-Pentyl-3-Methylimidazolium dihydrogen phosphate, usually described as an ionic liquid, is one of them. Lots of researchers prize it for its ability to dissolve tough compounds or act as an alternative to regular solvents. The question of safety isn't just academic; it shapes whether you keep your hands, eyes, and lungs safe.
Known Hazards and Underrated Risks
Some ionic liquids earn a reputation for low volatility, which cuts down on vapors and fire risk. Not all are the same, though. Experts in chemical safety, such as those at OSHA, warn that certain families may still irritate skin, eyes, and airways. With 1-Pentyl-3-Methylimidazolium dihydrogen phosphate, published research shows limited acute toxicity but little long-term data. It's easy to dismiss sticky, oily liquids as harmless if they don't have a strong smell or immediately feel irritating. That’s a mistake.
Chemical safety data sheets and peer-reviewed sources point to mild corrosivity and the potential to cause skin and eye irritation on contact. Absorption through broken skin isn't well defined, but no professional takes chances: gloves and goggles serve as non-negotiables when handling this chemical. Spilled on a bench or a hand, it leaves an uncomfortable film that sinks into creases, and standard soap and water may not cut it.
Regulatory Oversight and Gaps
Organizations like the European Chemicals Agency document tested hazards for many substances and recommend minimizing exposure. For some ionic liquids, there is a hope that they break down more easily in the environment than traditional solvents. With 1-Pentyl-3-Methylimidazolium dihydrogen phosphate, breakdown products and chronic effects in water systems aren't fully mapped. I have seen well-meaning colleagues assume “green solvent” status means “safe,” only to find out a few years later that extra clean-up steps are needed after spills.
Real-World Lab Protocols
In a crowded university lab, safety routines make the difference between a close call and a call to emergency services. Using 1-Pentyl-3-Methylimidazolium dihydrogen phosphate, everyone wears nitrile gloves, snug safety glasses, and lab coats with cuffs that keep liquids off the skin. No shortcuts. Fume hoods become a trusted ally—vapor pressure is low, but any unknown compound can aerosolize when heated or splashed.
Waste goes into clearly labeled containers for hazardous organic waste. There’s no dumping down the sink, even on the last day of a semester when everyone wants to leave early. Emergency procedures are printed at the station: splash in the eye, rinse under the eyewash; spill on the skin, flush for minutes.
Solutions and Forward Steps
Companies and universities should invest in up-to-date safety training for ionic liquids. Labs should add standardized signage and spill kits specifically for these chemicals, because guessing the right clean-up approach wastes time and risks health. Producers need to fill in the blanks with more toxicity testing. When new chemicals such as 1-Pentyl-3-Methylimidazolium dihydrogen phosphate enter regular use, their handling protocols deserve the same scrutiny as those for old-school solvents, no matter how promising their “green” credentials look on paper.
Hands-on experience, clear protocols, and a healthy suspicion for underexplored risks go further than a spreadsheet of test results. Safety grows out of habits, not labels.
The Everyday Puzzle of Product Storage
Every time I walk into my kitchen or open my medicine cabinet, the way items are stored jumps out at me. I've lost count of frozen vegetables turned limp, bottles of pills that clump together, or even snacks that go soft – all because they sat in the wrong spot. The experience goes beyond groceries. Manufacturers, distributors, and even local shops depend on storing products the right way to keep them useful, safe, and appealing.
Temperature: The Sneaky Spoiler
Let’s talk temperature. Heat wrecks more than taste. It can fuel bacteria, upset chemical balance, and change how medicine works. One study from the World Health Organization shows nearly 20 percent of vaccine shipments lose effectiveness due to improper temperature control. Cold chain breaches don’t just ruin food; life-saving medications like insulin or EpiPens become unreliable as soon as temperatures stray from what the label suggests. I’ve seen friends store their pharmacy pickups in hot cars, only to discover their medicine no longer works as it should.
Light and Moisture: Silent Trouble Makers
Direct sunlight once destroyed my favorite herbal tea blend. I left the pouch on my windowsill. The taste faded, the color dulled, and the smell vanished. That experience taught me about the power of light exposure. Light often breaks down fragile compounds in oils, vitamins, and supplements. Moisture does different damage, drawing in mold and clumping powders. A Harvard Medical School article describes how humidity above 60% shortens shelf life for tablets and capsules. If someone keeps probiotics or dried goods in the steamy kitchen, they’re playing a risky game with both money and health.
Safe Storage Is a Safety Issue
Many people overlook proper storage altogether. I once visited a small clinic where vaccines waited on a desk – close to the heater. The staff wanted convenience, but they were putting patients at risk. Something similar plays out daily in restaurants, grocery stores, even homes. Unsafe storage opens the door to bacteria, spoiled food, or wrong doses from degraded drugs. These risks don’t just mean fewer nutrients; they bring allergies, food poisoning, or even serious health emergencies.
Making Thoughtful Choices
Small, sensible fixes make a huge difference. I started using clear labels with purchase dates on containers, keeping food in dark, sealed bins, and storing medicine on higher, cool shelves away from sunlight. Businesses can benefit from reliable thermometers, regular checks, and better staff training. Even changing the spot where a box sits on the shelf can protect it from heat, drafts, or spills. It doesn’t take fancy technology to shield products from damage – just everyday vigilance.
No Shortcut for Care
The effort you put into storing a product reflects in its safety and usefulness. Each packet, bottle, or jar deserves respect. It’s not hard to keep salt dry, yogurt cold, or aspirin out of the sun. Small habits protect what matters. In the end, smart storage isn’t just about preserving money or meeting rules. It means delivering what people expect and need every time they open a package or reach for a meal.
The Stuff Behind the Label
Most of us never stop to check the fine print on chemical products, but those details about purity and grade carry real weight in how the world runs. My own brush with the wrong grade of ethanol during a college chemistry project taught a lesson I didn’t see coming. What looked like a standard bottle on the shelf turned out to contain trace contaminants that messed with our reactions. We laughed about it then, but in a hospital or a food plant, mistakes like that don’t end in jokes.
What “Purity” Actually Means
Purity sounds simple but opens a can of worms the moment you dig deeper. In chemistry, a “pure” substance is something made up almost entirely of one type of molecule, with unwanted stuff measured in tenths or hundredths of a percent. Scientists and buyers check lab reports and certificates to see those numbers, but not everyone understands what they mean. Raw chemicals from less reputable suppliers often have residues left behind from how they were made or stored. Pharmaceutical and lab settings watch for these traces because even small amounts throw off sensitive work.
The Different Grades: More Than Jargon
Grades sort chemicals for their intended uses. Reagent grade tops the list for labs. It’s clean enough to avoid messing with critical measurements. Technical grade works for concrete or factories where accuracy matters less. Food and pharmaceutical grades tell us the chemical has passed stricter impurity checks, protecting health where it counts. I visited a food processing plant last year where using the wrong salt could spark an audit or a recall. Those standards exist because history has shown time and again: shortcuts here can lead to disaster. The early 20th-century food adulteration scandals come to mind—people paid a steep price before the demand for food-grade regulations.
Why the Facts Matter
The FDA, EPA, ISO, and other agencies lay down requirements so businesses can’t just say something is “pure” and hope no one checks. They test random samples, ask for documentation, and sometimes even show up for a surprise look in person. I've seen enterprises lose importing contracts simply because they failed to show consistent test results and supply chain transparency. Reproducibility in scientific research depends on having known standards; without strict grades and reliable purity, labs risk publishing results that nobody else can repeat.
Making Better Choices
For buyers or researchers, the best step starts by demanding documentation. Reputable suppliers offer not only a certificate of analysis but also batch-specific test results and information on storage. Look for third-party audits and accreditations. If in doubt, a small investment in independent lab testing can save a lot of money and headaches later. In science and industry, trust develops over time—by working with suppliers who answer questions, stand behind their product, and don’t dodge tough conversations. That’s where everyone—patients, customers, and workers—win in the end.
Understanding the Risks and Realities
Anyone working in a lab, a pilot plant, or a manufacturing environment will cross paths with chemicals that don’t fit neatly into the “pour it down the drain” category. 1-Pentyl-3-Methylimidazolium Dihydrogen Phosphate sits among a growing family called ionic liquids, lauded for their low volatility and flexibility in chemical processes. Yet, like many specialty chemicals, its upside often gets all the attention while disposal receives a passing mention—usually at the end of safety data sheets. That does not line up with real-world responsibility.
Ionic liquids don’t evaporate into the air like traditional solvents, which looks green on paper. Down the line, they can linger in water or soil a whole lot longer. Studies like those published in Chemosphere have flagged their toxicity toward some aquatic organisms—not the highest out there, but not low enough to ignore, either. Environmental persistence remains a main concern because it isn’t just about today, but the next few years or even decades.
Legal and Social Obligations
In most regulated countries, dumping any unusual organic compound down the sink is a rookie error that can get you fined—or worse. Regulatory agencies, such as the EPA in the US and the Environment Agency in the UK, hold clear guidelines about hazardous waste handling and tracking. More and more, I notice companies not just fearing regulation, but wanting to do right by their staff, neighbors, and the environment. The moral calculus of responsible disposal lines up perfectly with best business practice. Throwing an ionic liquid like this one into the regular trash or sewer stream just doesn’t cut it anymore.
Practical Steps for Safe Disposal
Labs and plants need a game plan that starts long before the chemical hits the waste barrel. Storing liquids like this separately from acids, bases, and flammables cuts down on risky mix-ups. Spending a little extra on clear labelling pays off in zero confusion down the line.
Most operations find the best route is partnering with a licensed hazardous waste contractor. These companies know how to package, document, and ultimately destroy or recycle chemicals in line with national rules. Typically, they use incineration at high temperatures, which breaks down organic compounds safely—though it does require energy and investment.
Forward-thinking labs seek ways to minimize waste in the first place. Sometimes, the solution involves switching procedures to use less of these substances or reclaiming old batches for future work. Reduce, reuse, recycle isn’t just a cliché; with specialty chemicals, every milliliter makes a real difference. Education matters here, too—training staff on procedures helps avoid accidental spills or bad habits that could lead to trouble.
Community and Industry Responsibility
One-off decisions have a ripple effect in science and industry. That counts for chemical disposal as much as anywhere else. While regulators can lay down the law, it’s daily choices in real workplaces that ensure ionic liquids don’t end up harming local waterways or soil. Sharing experiences across labs and organizations, setting high internal standards, and supporting transparency help keep everyone in check.
We all want progress in science and technology, but not at the cost of health or the environment. Treating the disposal of chemicals like 1-Pentyl-3-Methylimidazolium Dihydrogen Phosphate as a shared obligation—rather than an afterthought—builds trust and reduces risk for everyone down the line.