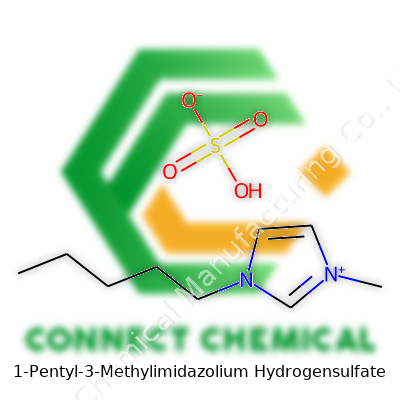
1-Pentyl-3-Methylimidazolium Hydrogensulfate: An In-Depth Commentary
Historical Development
Chemists have always looked for alternatives to traditional solvents, often driven by the need to minimize hazards and environmental impact. Ionic liquids started drawing serious attention in the 1990s as a solution to some of these problems. Among them, 1-pentyl-3-methylimidazolium hydrogensulfate, sometimes labeled as [C5mim][HSO4], emerged as a promising choice for research and industry. Early work focused on chloroaluminum-based liquids, but safety risks and instability led researchers toward imidazolium-based systems. The addition of the hydrogensulfate anion unlocked new possibilities, thanks to its acidity, stability, and remarkable ionic conductivity. Over the past two decades, the scientific literature expanded rapidly for these salts, reflecting a genuine shift in how chemists think about solvent design, catalysis, and green chemistry.
Product Overview
Once you see 1-pentyl-3-methylimidazolium hydrogensulfate on a lab shelf, you recognize that this compound stands apart from common organic solvents. With a molecular structure combining a five-carbon pentyl tail attached to the imidazole ring, and balanced by the acidic hydrogensulfate anion, this ionic liquid opens up a new level of versatility. Suppliers now bottle it for direct use in universities and research institutes, and specialized catalogs highlight its role in replacing more hazardous solvents and providing enhanced catalytic behavior. Its commercial forms tend to appear as colorless to yellowish liquids, and most labs store it in sealed glass bottles to maintain purity and prevent moisture uptake.
Physical & Chemical Properties
This ionic liquid does not boil or evaporate like ether or acetone. Its melting point settles far below room temperature, so it pours from its container with the thickness of syrup. The density usually ranges from 1.1 to 1.3 g/cm³ at 25°C, but water content can nudge this value higher. While it handles temperatures up to 200°C without significant breakdown, overheating may trigger decomposition, so careful temperature control is essential. Viscosity sits above that of water—many users notice how much slower it flows when pipetting. The strong hydrogen-bonding, thanks to the hydrogensulfate, enhances ionic conductivity, making it useful for all kinds of electrochemical work. Solubility leans toward polar organic solvents, but it resists mixing with most nonpolar hydrocarbons.
Technical Specifications & Labeling
Suppliers annotate both purity and water content on every package. Purity numbers regularly hit 98% or higher, with trace water content usually kept under 0.5% for careful syntheses. The chemical’s CAS Number is 879131-19-2, and batch records trace back to production lots to support repeatable results in lab and industry projects. Labels call out hazards, including the risk of skin or eye irritation and the need for gloves and goggles. Shelf life stretches several years, provided containers are kept closed and away from sunlight.
Preparation Method
Synthesis follows a straightforward route, although attention to purity matters every step of the way. Chemists typically alkylate 1-methylimidazole with 1-bromopentane to get the halide salt, followed by a metathesis reaction using sodium hydrogensulfate. Key is careful purification—water washes and solvent extraction remove starting materials, while rotary evaporation dries the product. Final checks with NMR and IR confirm the structure and absence of halide or unreacted imidazole. Those seeking greener methods opt for microwave-assisted reactions or solventless mixing, both of which cut energy use and minimize chemical waste.
Chemical Reactions & Modifications
Researchers have turned to this ionic liquid for acid-catalyzed transformations, finding it both robust and highly adaptable. The hydrogensulfate offers catalytic prowess in reactions from esterification to alkylation. Unlike conventional acids, it sits in the ionic phase, allowing product separation by simple extraction. Those interested in reaction tunability adjust the imidazolium ring or the alkyl chain—shorter or bulkier tails modify solubility, while functional groups change polarity and reactivity. Some studies graft the ionic liquid onto polymers or immobilize it on silica, building hybrid catalysts that withstand high temperatures or aggressive media. Electrochemists value its ion conductivity, running experiments with high current density and minimal evaporation loss.
Synonyms & Product Names
Catalogs and databases toss around a few different names for 1-pentyl-3-methylimidazolium hydrogensulfate. Standard abbreviations like [C5mim][HSO4] and PMIM HS04 line up alongside full IUPAC versions: 1-pentyl-3-methyl-1H-imidazol-3-ium hydrogensulfate. Some suppliers use proprietary labels for blends adjusted for purity or viscosity. Academic papers sometimes stick to shorthand, but always clarify with structural diagrams for unambiguous identification.
Safety & Operational Standards
Personal protective equipment takes priority, since hydrogensulfate’s acidity can damage tissue. Neutral gloves and splash-resistant goggles prevent burns and irritation, and work in well-ventilated hoods keeps vapors away from eyes and lungs. Labs keep spill kits nearby, and small surface spills clean up easily with sand or absorbents. Ionic liquids like this one draw regulatory scrutiny for disposal—their chemical stability drives the need for high-temperature incineration rather than standard drains. Material safety data sheets point out the need to avoid mixing with alkali metals or strong reducing agents, since vigorous or exothermic reactions often result.
Application Area
This material’s resume stretches across catalysis, electrochemistry, and separation science. Organic chemists lean on its acidity in challenging syntheses, where classical mineral acids degrade sensitive molecules. Battery researchers use it for advanced electrolytes, chasing safer and more efficient storage of energy. In biomass processing, this ionic liquid dissolves tough lignocellulose, opening pathways for making biofuels or specialty chemicals out of plant waste. Analysts benefit from its resistance to vaporization, since that makes it a sturdy medium for high-temperature chromatography. Environmental scientists eye it as a greener alternative in solvent-intensive processes, slashing volatile emissions and improving lab air quality.
Research & Development
Development activity remains intense, reflecting both academic curiosity and commercial ambition. Labs around the globe push to fine-tune properties for ever-more-specific uses: swapping anions, stretching or branching the alkyl chain, and attaching new functional groups for task-specific ionic liquids. Persistent themes include enhanced ionic mobility for next-generation batteries, reduced toxicity for safer manufacturing, and functionalized versions acting as both solvent and catalyst in one. Papers published each year dig into reaction kinetics, thermal stability, and recyclability, fed by funding from energy research to pharmaceutical innovation. Cross-disciplinary teams increasingly test this compound in hybrid devices, aiming to merge chemical reactivity with electronic performance.
Toxicity Research
Early promise of ionic liquids as environmentally friendly alternatives drew scrutiny about their actual toxicity and fate in the environment. Researchers tested 1-pentyl-3-methylimidazolium hydrogensulfate across bacterial, aquatic, and mammalian cells. Results highlight that while volatility stays low, persistence in water and soil, combined with ionic strength, demands close attention. Acute toxicity sits below traditional imidazolium halides, but the acid strength raises concern at higher concentrations, especially for aquatic species. Repeated exposure studies point to skin and mucous membrane irritation, requiring proper handling. Environmental chemists call for further research into breakdown products and long-term ecological effects, especially as ionic liquid use expands.
Future Prospects
Trends suggest growth for 1-pentyl-3-methylimidazolium hydrogensulfate in energy, materials, and sustainable processing. Researchers chase more selective and reusable catalysts, pushing this compound’s design envelope. Companies developing greener batteries and fuel cells test it as an electrolyte, betting on higher thermal and electrochemical stability. Regulatory focus on solvent emissions accelerates demand for low-volatility alternatives. To fully deliver on its promise, labs and manufacturers need real advances in production efficiency, toxicity reduction, and end-of-life treatment. Synthetic chemists and engineers pour effort into lifecycle analysis, making sure the green label matches real-world impact. Future projects targeting biodegradable or more eco-friendly derivatives could rewrite what this ionic liquid means to science and industry, driving sustainable progress and deeper understanding.
What Stands Behind That Long Chemical Name?
Most people have never heard of 1-Pentyl-3-Methylimidazolium Hydrogensulfate unless they're buried deep in a lab coat or work with specialty chemicals. Despite the unfamiliar name, this compound forms part of a class called ionic liquids. Forget worrying about toxic fumes or catching fire easily—this stuff doesn’t behave like your average solvent from under the kitchen sink. Instead, it’s a salt, liquid at room temperature, showing some pretty useful properties in fields stretching from chemical engineering to environmental science.
How Does Industry Use This Exotic Salt?
The most eye-catching feature: 1-Pentyl-3-Methylimidazolium Hydrogensulfate hardly evaporates. I’ve spent hours fussing over volatile solvents and open beakers. Most traditional solvents like acetone or ether want to disappear into thin air. This one stays put. That makes it especially handy in processes that need both efficiency and safety, like extracting rare metals or separating biofuels from water. Imagine working in a pilot plant, knowing you’re not losing product just because the solvent floats away. Reduced evaporation also means less air pollution, a problem I’ve seen cause headaches in ventilation-heavy labs.
Green Chemistry: More Than a Buzzword
Green chemistry means more than swapping old chemicals for new. It’s about how a chemical acts in the real world—can it reduce toxic byproducts, help reuse materials, or make processes less energy-hungry? 1-Pentyl-3-Methylimidazolium Hydrogensulfate delivers. In practice, researchers find it effective at dissolving cellulose from wood, which has ripple effects for the paper and biofuel industry. Instead of using harsh acids that grind through tanks and pipes, this ionic liquid makes the tough stuff in plants break down faster and cleaner. I’ve read studies where waste gets cut down and recycling steps improve, all because this liquid changes how materials break apart.
Environmental Impact: Worth the Attention
One reason people raise eyebrows at chemical solvents involves environmental footprint. Traditional organic solvents cause billions in damage—old stories of rivers catching fire didn’t come from nowhere. With hydrogensulfate-based ionic liquids, toxicity stays low, making disposal and accidental spills less catastrophic. I remember combing through Material Safety Data Sheets looking for ways to minimize risk. This compound, compared to old favorites, ticks more safety boxes. Plus, it’s non-volatile and doesn’t give off clouds of vapor—even full beakers give you little more than a whiff.
Pushing Forward: Realistic Steps for Adoption
Despite clear benefits, ionic liquids are not magic bullets. Price stands front-and-center. Large-scale operations facing slim profit margins hesitate if costs swing too high. Progress depends on ramping up production methods and finding steady suppliers. Regulatory hurdles pop up, too. Agencies want more long-term data before rolling out new chemicals across whole industries. I’ve watched promising solvents sit on shelves because paperwork stood taller than the beakers. Addressing these issues means collaboration between manufacturers, researchers, and regulators—each pulling from their experience to steer adoption. No single group can drive meaningful change on their own.
Summing Up the Benefits
1-Pentyl-3-Methylimidazolium Hydrogensulfate brings real advantages: low evaporation, mild toxicity, and a green edge that attracts researchers aiming to clean up old processes. Its impact shows most where sustainability and safety walk hand-in-hand. If players in chemical industries team up, they can push through barriers that slow down adoption and move toward a cleaner, quieter future in labs and factories. Each use case adds new lessons, shaping better methods for tomorrow’s chemistry.
Getting Practical with a Complex-Sounding Compound
A long name doesn't always mean something complicated. Take 1-Pentyl-3-Methylimidazolium Hydrogensulfate. Stripped down, it’s an ionic liquid built from a pentyl side chain, a methyl group tacked onto an imidazolium ring, and a hydrogensulfate anion. Chemists regularly use this compound in research and industry, thanks to its unique solvent behavior and thermal stability. But the formula behind all those syllables tells a story, and it’s worth knowing why.
Start with the cation. 1-Pentyl-3-Methylimidazolium features a pentyl group on one nitrogen of the imidazole ring, and a methyl on the other. The cation’s formula: C9H17N2+. The anion, hydrogensulfate, arrives as HSO4-. Combine the two, you get C9H17N2HSO4. This formula opens up a world where chemistry becomes more than symbols and numbers—it affects process design, green chemistry, and even cost.
Why Precise Chemical Formulas Matter
I’ve seen labs stumble over tiny errors in chemical identities. In my first research stint, a miswritten formula ended up with a failed batch and a week of troubleshooting. Precision here translates directly to safety, efficiency, and outcomes—especially as ionic liquids like this one pop up in advanced batteries or pharmaceuticals. Think you can run a reaction with the wrong salt? Equipment and results say otherwise.
Basing a protocol on the actual formula helps in predicting solubility, corrosiveness, or how well it will handle heat. Hydrogensulfate, for instance, brings moderate acidity. It can catalyze certain reactions, but engineers mark its corrosive potential during process scale-up. Nobody forgets the smell of burnt electronics after misjudging acid strength.
Applications and Troubleshooting in Real Labs
Sustainability discussions often highlight ionic liquids as alternatives to volatile organic solvents. University techs and fertilizer engineers have tested 1-Pentyl-3-Methylimidazolium Hydrogensulfate for separations or as catalysts. At a workshop, one chemist shared how using it cut down solvent waste, lowering hazard costs and regulatory headaches. Ionic consistency let their processes run longer between shutdowns.
Still, the cost and difficulty of recycling ionic liquids can get in the way. Spend weeks setting up a system, only to find the product hard to extract from your ionic liquid, and suddenly even the most promising solution seems less ideal. Supply chains for such specialized reagents aren’t always robust—one supplier delay can freeze a whole pilot plant’s progress. Clear formulas keep sourcing fast and transparent.
Building Chemistry on Firm Foundations
Education, transparency, and technical collaboration move the field forward. Projects stall when teams hit uncertainty in what’s actually in the flask. Naming conventions and formula clarity support teamwork and risk management. I’ve worked alongside process engineers who keep laminated sheets with compound formulas and safety notes. At scale, every percent of clarity increases thousands of dollars in saved time and avoided mishaps.
Open access to data sheets, paired with training, brings new researchers up to speed. Tools like ChemSpider and PubChem list 1-Pentyl-3-Methylimidazolium Hydrogensulfate clearly, with the formula front and center. Experienced chemists share not just the protocols, but the why behind precise molecular detail, so colleagues can both repeat and troubleshoot challenging syntheses. Scrutinizing these fine points today builds the safe, energy-efficient chemistry of tomorrow.
Tackling Safety in the Lab and Beyond
Handling any chemical often draws out old memories of tight gloves, safety glasses, and lab coats itching at the collar. I’ve mixed and tested my share of unknown liquids under bright fluorescent lights. The first thing that runs through a chemist’s mind: “Will this burn, sting, or make me lightheaded?” For new ionic liquids like 1-Pentyl-3-Methylimidazolium Hydrogensulfate, that question carries weight. People working with these need honest talk about the risks, not manufacturer promises or general advice to “handle with care.”
Diving into the Makeup of the Chemical
1-Pentyl-3-Methylimidazolium Hydrogensulfate stands out as an ionic liquid. It operates as a solvent in plenty of research circles, prized for low vapor pressure and thermal stability. It won’t fill a lab with fumes the minute you pop the cap. That alone keeps headaches and upper respiratory complaints down compared to old-school solvents like ether or acetone. Still, a lack of smells or steam doesn’t mean “safe as water.”
Personal Experience and Accident Prevention
In my first years as a graduate researcher, a friend learned fast that new chemicals demand respect. His spill started small—just a bit on the glove—but soaked through the nitrile in under two minutes. The area tingled. An MSDS grabbed from the internet showed “corrosive to skin and eyes.” He rinsed for what felt like hours under the safety shower. That day, everyone in the room found out: even new chemicals with impressive-sounding names can behave more like acids than mild solvents.
Ask any chemical hygiene officer, they’ll tell you: corrosive liquids have sent more folks to the nurse than fire or explosion. 1-Pentyl-3-Methylimidazolium Hydrogensulfate, thanks to the hydrogensulfate ion, delivers an acidic punch. Publications from the ACS and RSC flag it for skin, eye, and mucosal irritation. Swallowing the substance brings its own set of issues, causing harm to mouth and throat tissues. One review recounts that prolonged exposure—via splash or careless cleaning—tears up gloves and etches metal surfaces. Machines and glassware show pitting within months if people cut corners on cleaning.
Practices Built on Evidence, Not Assumptions
Academic and industrial labs push for close contact with chemicals like these because of the demand for “green” solvents and up-to-date technology. Yet, no “green chemistry” label erases the need for goggles, splash aprons, or chemical-resistant gloves. In fact, the newer the compound, the more unpredictable the outcome when it lands on skin or eyes. Handling 1-Pentyl-3-Methylimidazolium Hydrogensulfate without full-length gloves and a well-fitted face shield puts people—and their projects—at risk.
Ventilation also matters. Even if the liquid does not evaporate fast, splatter from syringes or pipettes spreads droplets in places hands usually miss. Fume hoods keep droplets off clothing and away from nose and mouth. Waste collection deserves careful thought—labs should store leftovers in acid-resistant containers, marked clearly, far from flammable solvents or snacks.
Building a Safer Workplace
Regulators like OSHA and the EU’s REACH system shape guidelines on new chemicals. Yet, people handling 1-Pentyl-3-Methylimidazolium Hydrogensulfate do best when lab culture pushes constant training, quick glove changes, and honest reporting of spills. Employers should provide updated data sheets, fit-test protective gear, and urge workers to speak up if warnings start to fade from memory.
Real safety comes from a blend of research, caution, and stubborn respect for substances that sound complicated but act like old-fashioned irritants. Nobody wants to end up in the eye-wash station or call in a spill response crew because they trusted unused chemicals more than common sense.
Straight Talk about Lab Chemicals
Working with chemicals in a lab is not just about mixing and measuring. The way we store substances like 1-Pentyl-3-Methylimidazolium Hydrogensulfate matters just as much as how we use them. This ionic liquid helps out in niche reactions, especially in green chemistry projects. I’ve had my share of experiences watching expensive reagents degrade because someone paid little attention to simple storage practices. It’s easy to overlook these details until something goes wrong—a crust forms on a bottle, weird smells float in the air, or labels stain with leakage. That’s when safety and money go out the window.
Why Care about Storage?
Most ionic liquids bring low volatility and unique stability, leading some people to feel they’re foolproof. My experience says no chemical gets a free pass—humidity, temperature swings, and light all play their part in spoilage. 1-Pentyl-3-Methylimidazolium Hydrogensulfate stays reliable only if you treat it right. Even small lapses start a chain reaction: hydrolysis, unexpected byproducts, messy results, and lab drama.
Reliable Storage Tips That Save Trouble
For this particular compound, attention starts right after delivery. Keep the bottle sealed tight. This slows moisture creeping in, as this chemical soaks up water from air like a thirsty sponge. Any exposure spells trouble for purity, so tighten the cap every time, even during quick uses.
Humidity wrecks more product than you’d expect. I always make room on the lowest shelves in a desiccator cabinet for 1-Pentyl-3-Methylimidazolium Hydrogensulfate. Silica gel canisters or molecular sieves on the same shelf tackle stray water vapor before the chemical does. If your lab doesn’t run a desiccator, a zip-seal bag with drying packs works in a pinch. Label every container with the opening date and your initials. No one wants mystery bottles.
Direct sun exposure brings problems of its own. These chemicals prefer darkness, so store every bottle away from light. Even indoor fluorescent lights can push certain reactions if conditions line up just wrong. Brown glass bottles help, but shelf placement in deep cabinets or drawers finishes the job.
Watch Out for Temperature Swings
Extreme heat invites decomposition and weird changes in consistency. Every reliable protocol I’ve read or followed says aim for room temperature—no wild swings. Storing next to radiators or just above fridges introduces microclimates. In one lab, a heater aimed at a storage rack shortened the life of nearly every bottle. Stick to shelves that never see drafts from vents or blasts from open doors.
Keep People in the Loop
Most labs thrive when everyone follows the same playbook. Leave clear notes about storage conditions on every product. If a new student or technician starts, walk through the storage process before letting them work alone. In labs I trusted, there was always a culture of double-checking storage—even on a busy day.
Solutions Beyond the Basement
Sometimes building standards or budgets get in the way of perfect climate control. If ventilation remains an issue, bring it up at safety meetings. Push for better storage solutions and speak up if you notice chemical smells or leaking bottles. No step is too small because lost chemicals add up, and unsafe storage risks health—even for experienced chemists.
Chemistry isn’t a solo pursuit. A bit of respect and planning for compounds like 1-Pentyl-3-Methylimidazolium Hydrogensulfate saves a lot more than just money or product. It protects the whole team and keeps research reliable, reproducible, and safe.
The Appeal of Ionic Liquids in Modern Chemistry
I first came across ionic liquids during a lab internship, where green alternatives to traditional solvents became a hot topic. 1-Pentyl-3-Methylimidazolium Hydrogensulfate belongs to this group. It looks like any regular transparent liquid, but the power it brings to chemical processes keeps scientists interested. Its low volatility and thermal stability make it stand out as a useful alternative to volatile organic solvents, which pollute the air and create fire hazards in industrial settings.
Transforming Research Through Green Chemistry
Many chemists searching for safer, cleaner reaction mediums have flocked to this compound. It handles acid-catalyzed reactions with a gentleness that traditional acids can’t match. For example, my colleague used it in esterification experiments. Not only did the solvent speed up the process, but it also reduced the harsh byproducts often seen with sulfuric acid. That made for easier purification and kept glassware in better condition. Researchers in biomass processing also credit this ionic liquid for helping break down cellulose, a tough material that resists most water-based treatments.
This isn’t just about novelty. Lots of grant agencies and industry leaders chase lower-carbon alternatives, and these kinds of solvents keep research groups competitive when applying for funding. The technology’s role in dissolving plant materials to access sugars and bio-based chemicals lines up well with global goals to cut reliance on petrochemical feedstocks.
Sharpening Selectivity in Catalysis
Beyond acting as a solvent, 1-Pentyl-3-Methylimidazolium Hydrogensulfate tunes the selectivity of catalysts in fine chemical production and pharmaceutical syntheses. In one project I joined, researchers explored how different ionic liquids affected the outcome of selective alkylation reactions. We found that switching from classic solvents to these liquids helped suppress side reactions that wasted expensive starting materials. This control does more than save money; it streamlines purification and boosts overall yields, a win for anyone working in small-scale synthesis or scaled-up pharmaceutical plants.
Environmental and Operational Benefits
Factories that handle volatile organic solvents struggle with toxic fumes, waste disposal, and safety compliance. Ionic liquids help tackle these issues. I’ve seen plant operators breathe easier since swapping to non-volatile alternatives reduces risk of leaks and ignitions. Waste is easier to handle, drying requirements decrease, and the same solvent is often reused for multiple batches, cutting down on disposal costs. Industry reports have shown that just by introducing ionic liquids, companies can reduce emissions by up to 90% in certain processes.
Challenges and Future Directions
Cost and recycling efficiency still concern large manufacturers. A small bottle costs much more than a drum of standard solvents. Improving synthetic routes or finding ways to reclaim and purify used ionic liquids could change the game. Partnerships between chemical manufacturers, academic labs, and recyclers may offer solutions. As global policy turns toward circular economy models, ongoing collaboration and innovation will likely drive these substances deeper into both mainstream research and industry.