1-Pentyl-3-Methylimidazolium Iodide: Commentary on Its Significance and Outlook
Historical Development
The road to 1-Pentyl-3-Methylimidazolium Iodide started with a surge of interest in ionic liquids back in the late 1980s. Chemists chasing greener solvents stumbled onto imidazolium-based compounds, realizing their solvent power stretched well beyond water and traditional organics. This molecule, with its pentyl tail and methyl tweak on the imidazole ring, came together as researchers tuned ionic liquids to dissolve stubborn reactants, ease separation, and lower environmental burdens. Lab work in Europe and Asia soon pinpointed the advantages of this specific iodide salt, helping open up avenues in material science and catalysis that old-school solvents struggled to address.
Product Overview
1-Pentyl-3-Methylimidazolium Iodide falls under the family of room-temperature ionic liquids. This compound usually appears as a yellowish solid or viscous liquid, depending on storage temperature and water content. Structurally, its bulkier pentyl group stands out against shorter-chain relatives, giving it unique solubility and viscosity. Users in research labs and specialty manufacturing value this ionic liquid for pairing both strong ionic character and the ability to mix with many polar and nonpolar substances. The iodide counterion introduces distinct reactivity, making this product more than just a laboratory curiosity.
Physical & Chemical Properties
The physical traits drive the use cases—1-Pentyl-3-Methylimidazolium Iodide tends to melt around 50°C, but impurities and hydration drop that threshold. Above room temperature, the material flows with honey-like consistency, and exposure to air pulls in enough water to shift its melting point. It dissolves in solvents like acetonitrile, DMSO, and water, opening doors for battery electrolytes, electrochemical cells, and organic transformations. The compound’s thermal stability stretches past 200°C, outlasting many alternatives. This ionic liquid resists decomposition under most lab conditions, unless strong nucleophiles or harsh reductants step in.
Technical Specifications & Labeling
Bottles from reliable suppliers typically bear purity levels over 97%, with minor traces of chloride, bromide, or other imidazolium salts monitored by HPLC or ion chromatography. The main identifying numbers—CAS and EC—help users avoid cross-contamination with closely related molecules. The labeling emphasizes storage in tightly sealed amber bottles, generally under an inert atmosphere, away from light and moisture. Specification sheets often highlight absence of volatile organic impurities, as well as guaranteed iodide content, since too much halide variation shifts chemical performance in batteries, catalysts, and sensors.
Preparation Method
The most practical syntheses use 1-methylimidazole and 1-pentyl iodide as building blocks. In practice, chemists blend these reagents in dry acetonitrile, running the reaction over a few hours at mild heat while stirring. Reaction vessels need to keep out water, as it poisons yield by encouraging side products like HI or di-alkylated imidazoliums. As the reaction wraps up, precipitation with ether and washes with hexane help remove excess starting materials. The purified crude solid dries under high vacuum. Some labs finish off with recrystallization from ethanol. This straightforward method bypasses hazardous strong acids and minimizes waste.
Chemical Reactions & Modifications
The imidazolium ring allows plenty of room for experimentation. Swapping out the iodide for other anions—PF6, BF4, NTf2—gives new solubility and reactivity profiles, suiting everything from organic synthesis to electrochemical gadgets. The pentyl tail shapes how the molecule packs together, affecting viscosity and ion conduction in devices. Some groups have alkylated the ring even further, targeting custom tail lengths or branching for improved solubility in oils or bio-compatible systems. Inorganic chemists sometimes use this iodide salt as a halogen-transfer agent for synthesis or nanomaterial templating. In my own stints in the lab, I’ve seen this salt serve as both electrolyte and reactant, morphing from pale yellow powder in a jar to deep red solutions as it chelates metals or exchanges iodine for stronger ligands.
Synonyms & Product Names
References to this chemical often use shorthand like C5MIM-I, 1-(Pentyl)-3-methylimidazolium iodide, or abbreviations like [C5mim][I]. Each lab, supplier, or catalog might cycle through versions, though the underlying ring-tails-and-iodide structure stays constant. In the market, researchers searching for this product often encounter names that reflect the N-alkyl group or emphasize the ionic liquid nature—terms like “alkylimidazolium iodide ionic liquid” or “pentyl-methylimidazolium iodide” pop up on labels and batch notes.
Safety & Operational Standards
Lab veterans pay special attention to the potential for eye and skin irritation, inhalation risks when handling powdered forms, and the hygroscopic nature of the material. Safety sheets from experienced manufacturers call for double nitrile gloves, splash goggles, and use in well-ventilated hoods. This compound doesn’t combust easily, nor does it give off flammable vapors, but its iodide raises concern for thyroid disruption with chronic exposure. Disposal usually means transferring all waste to halogenated solvent streams for incineration, sidestepping environmental buildup. Long-term storage in desiccators or gloveboxes keeps both the product and its users safer, since hydrated, degraded samples clog up microfluidics or batteries.
Application Area
Use cases for 1-Pentyl-3-Methylimidazolium Iodide stretch into energy storage and green chemistry. Laboratories favor it in dye-sensitized solar cells, where it acts as a solid-state electrolyte, improving both charge transport and stability. Catalysis groups press it into service for phase-transfer or media-switching: its ionic character stirs up sluggish oxidations or enables cross-coupling in unconventional solvents. Material scientists lean on this salt to stabilize nanoparticles or facilitate membrane self-assembly. In electrochemical setups, I’ve seen this material bridge the gap between low conductivity and high reactivity, especially when tracking ion flow in prototype cells. Med chem researchers take note of the alkyl tails—changing their length can swing pharmacological properties widely, highlighting the role these imidazolium salts play in drug formulation screening.
Research & Development
Innovation in the field doesn’t slow. Research labs publish about tweaking both the imidazolium ring and the anion component, showing fresh results for reaction yields, device lifetimes, or separation efficiency. Work in Japan and Germany investigates using this iodide salt to replace carcinogenic solvents in pharmaceutical synthesis. Chinese university spinoffs focus on tailoring this molecule for antistatic coatings and organic electronics. My own reading has revealed a steady stream of patents on using this compound for carbon dioxide capture, where the iodide plays a part in binding or activating greenhouse gases. Upstreams providers push for greener manufacturing, aiming to phase out old halogen sources and recycle spent ionic liquids, closing the loop.
Toxicity Research
Early work flagged some concerns—ionic liquids aren’t always “green” by default. The pentyl tail boosts fat solubility, raising the odds of cell membrane disruption if this salt spills into the environment. Toxicity studies on aquatic worms and freshwater fish report bioaccumulation and enzyme inhibition at moderate concentrations. Human safety data lags behind, but medical journals warn about chronic iodide exposure and possible thyroid effects if employees mishandle the salt day after day. Most academic and industrial protocols now aim to reduce open handling, rinse contaminated glassware in halide-neutralizing baths, and work up reactions behind shields. In my years teaching chemical hygiene, I hammer home that “ionic” never equals “inert”—if anything, it’s the opposite.
Future Prospects
Momentum keeps building. Researchers look at the next generation of ionic liquids, balancing task-specific performance with lower toxicity and full recyclability. With energy storage and solar tech hungry for robust, stable materials, 1-Pentyl-3-Methylimidazolium Iodide takes its place as both target and template. Companies scale up biobased or less hazardous alternatives, and regulators set stricter turnover rates for ionic liquid residues. Still, continuous improvement stands as the main path—smarter synthesis, rigorous safety, and life-cycle analysis for every bottle shipped or gram recovered from waste streams. The challenge isn’t just invention, but stewardship, making sure each new application harmonizes with workplace safety, supply chain security, and long-term environmental health.
A Powerhouse in Solar Cell Research
People searching for new sources of energy have always looked for better ways to harness sunlight. One material making noise in this arena is 1-pentyl-3-methylimidazolium iodide, which finds its main purpose as an ionic liquid in dye-sensitized solar cells (DSSCs). These cells compete with more common silicon-based panels but get the edge from the special mix of chemicals at their core. In my chemistry days, we kept hearing stories about how ionic liquids like this gave DSSCs more muscle through better electron transport and strong thermal stability. Scientists racing to build better solar tech keep gravitating to this molecule for good reason.
How It Makes a Difference
Regular DSSCs can suffer from quick loss of liquid electrolyte or quirky changes under temperature swings—just what you’d expect if your technology uses standard solvents. By swapping in 1-pentyl-3-methylimidazolium iodide, researchers get a salt that acts like a solvent but doesn’t evaporate easily. It lasts under stressful lab situations, which matters if you want a solar cell that survives summer on a rooftop. Some numbers really speak for themselves: DSSCs using this ionic liquid have hit efficiencies above 7%, with fewer hiccups from temperature jumps.
The story doesn’t end with stability. I’ve seen research labs line up vials of different ionic liquids and watch, with a mix of hope and frustration, as only a couple of candidates really push the needle on performance. Hydrogen bonding, viscosity, and chemical compatibility all play their part, and the pentyl chain in 1-pentyl-3-methylimidazolium iodide helps keep redox reactions moving, letting electrons flow with less backtracking—or what scientists call recombination.
Environmental and Practical Challenges
The push for renewable energy leads to inevitable questions about the green credentials of each new material. Being an ionic liquid, this compound doesn’t let off volatile organic compounds like some other options, helping indoor labs and future factories stay cleaner. But I have yet to see a clear answer on long-term environmental effects after these solar cells reach the end of their life. The imidazolium part of the molecule can break down under tough conditions, and waste management for ionic liquids has lagged behind their discovery and use.
Room for Improvement and the Path Forward
People working on next-generation DSSCs often call for a deeper look at how these ionic liquids interact with other cell components. Some researchers tweak the alkyl chain or swap out the iodine for other halides to push current and voltage even higher. Still, scaling up always brings fresh roadblocks—sourcing these chemicals at scale or guaranteeing purity for mass production takes time and effort.
From what I’ve observed, interest grows fastest where government funding or industry partnerships step in. Pioneering university labs keep testing new designs but rarely shift to mass production without support. Training chemical engineers to recycle or safely dispose of spent ionic liquids will become part of widespread adoption if these solar cells break out beyond the lab bench.
The story of 1-pentyl-3-methylimidazolium iodide serves as a snapshot of broader trends in materials science: one chemical, carrying the hopes of cleaner energy, but not without costs and new questions. Its journey in the solar world has just begun.
Understanding What You’re Working With
1-Pentyl-3-methylimidazolium iodide falls under the family of ionic liquids, a group of salt compounds that stay liquid at lower temperatures. This stuff has gained a lot of interest for uses in batteries, dyes, and organic synthesis. It doesn’t mean it’s as safe as water, though. You can’t take shortcuts just because it’s not a strong acid or base. The way this compound interacts with living tissue and the environment makes it something to take seriously.
The Risks That Matter
Getting information about the real health risks of 1-pentyl-3-methylimidazolium iodide isn’t always easy. What we do know points toward the common sense steps you’d use with any lab chemical that hasn’t been fully tested for chronic or acute toxicity. Most imidazolium-based salts pose risks from inhalation, skin absorption, and possible eye injury. Even if nobody drops over from a splash, chronic exposure could build trouble over time. Data from similar chemicals suggests potential irritation, allergic reactions, and interference with cellular processes. This info usually comes buried in journals, but ask any researcher who has spilled a new ionic liquid and watched their gloves pucker up—it’s a reminder that the unknowns demand respect.
Practical Precautions in the Real World
Old school chemists often worked with benzene without gloves; not surprisingly, many faced health issues later. With learning comes better habits. Lab gloves—nitrile, not latex—give a reliable barrier for most ionic liquids. I’ve seen folks think they’re fine because there’s no obvious burn, then show up with a rash a week later. Splash-proof goggles and long sleeves matter, too. Some ionic liquids chew through watchbands, so covering your wrists is sensible. Ventilation plays a big role—if you haven’t set up a proper fume hood, don’t try to get creative near open windows. Liquid spills on the benchtop should get neutralized with something absorbent followed by a wipe-down using soap and water, not just a paper towel swipe. If you ever find yourself unsure, cleaning twice beats ending a day with skin irritation or eye redness.
The Duty to Others and the Planet
Beyond personal health, consider where leftovers and contaminated materials end up. Ionic liquids sometimes get tagged as “green” in marketing because they don’t evaporate easily, but that doesn’t give them a free pass. Some break down slowly in the environment, potentially releasing harmful bits as they go. Proper waste disposal is not just a box-ticking exercise. Spill kits, clearly labeled collection flasks, and a habit of cleaning workspaces before each session create a culture of care.
Getting It Right Every Time
Labs can be busy and messy, with people rotating on and off projects. Sometimes the biggest risk comes from someone grabbing a bottle and not double-checking the label. I keep a habit of logging every use and flagging anything unusual—strange smells, odd colors, glove problems. No one likes paperwork, but ten seconds now can prevent a panic later.
It’s tempting to push caution aside to save time, especially under pressure. I’ve never run into a scientist who regretted taking one more minute for safety, but I’ve known a few who still carry the scars from times they didn’t. Treat 1-pentyl-3-methylimidazolium iodide like any unfamiliar chemical: keep your barriers up, keep your workspace clean, and never trust a “harmless” reputation over your own routine of double-checking. This habit, not luck, keeps people working, learning, and coming home safe every day.
The Basics Behind the Name
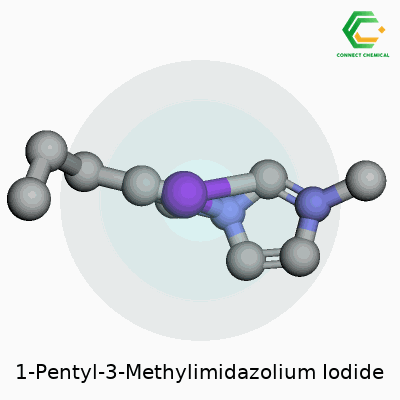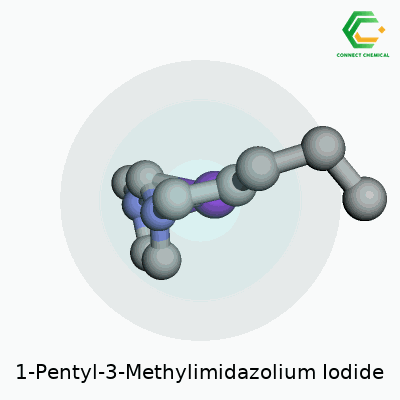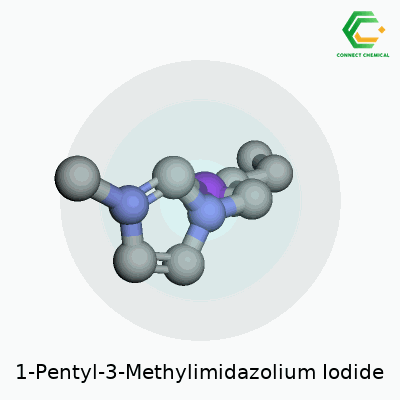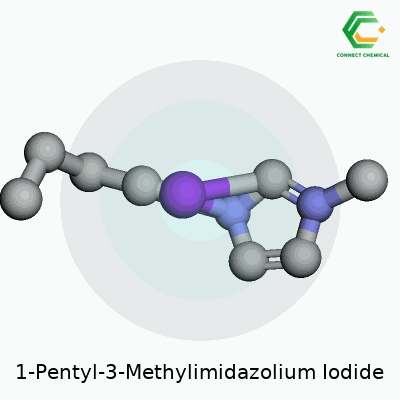
Chemistry can sound complicated, especially once names like 1-Pentyl-3-Methylimidazolium Iodide get thrown around. Take the name apart, and you get a picture of how the molecule is built. The core is imidazole, a ring-shaped structure made of three carbon atoms and two nitrogens. Add a methyl group to the third nitrogen—picture a simple “branch” with a carbon and three hydrogens sticking out. The pentyl group tacks onto the first position, a straight chain of five carbons. The molecule picks up a positive charge here, forming an imidazolium cation. Partner this with an iodide anion, and the ionic liquid forms.
Chemical Structure and Formula
The chemical formula comes out as C9H17N2I. Count up each element: nine carbons from the chain and the ring together, seventeen hydrogens from the various branches, two nitrogens from the ring, and the iodine from the anion. Chemists visualize the structure as a five-membered ring, where the nitrogens grab electrons and shape how the molecule interacts with its environment.
Draw the structure on paper, and it looks like this: the imidazole ring has nitrogens at positions one and three. The pentyl chain attaches to nitrogen one, the methyl group goes to nitrogen three. The iodide ion floats nearby, balancing out the positive charge carried by the imidazolium.
Practical Importance
The formation of these imidazolium-based ionic liquids changed the way researchers think about solvents. Regular solvents evaporate, sometimes making labs stink or risking worker health due to fumes. Ionic liquids reduce that problem. Their low volatility makes them useful as “green” solvents in labs that care about indoor air quality and reduce risk of fire. The structure of 1-Pentyl-3-Methylimidazolium Iodide means it can dissolve a lot of different compounds, including some that traditional solvents struggle to handle.
Where the Facts Lead
According to several published studies in journals like Green Chemistry and ChemSocRev, imidazolium-based ionic liquids have earned a place in chemical research for their unique properties. They conduct electricity, making them handy in dye-sensitized solar cells, certain batteries, and organic synthesis. The C9H17N2I formula isn’t just a random string of letters and numbers. It signals potential in pharmaceutical research, catalysis, and even carbon capture, turning abstract research into practical solutions for pollution and energy storage.
Issues and Possible Improvements
While these compounds look promising, production costs and environmental fate remain hurdles. Making ionic liquids still requires specialized reagents, which pushes the price higher than everyday solvents like ethanol. Research groups around the world keep searching for cheaper, easier ways to manufacture these salts. Scientists also investigate how ionic liquids break down in the environment. Some stick around longer than they should, raising questions about persistence and toxicity. Revising the alkyl chain length or swapping the anion could address these concerns—early evidence suggests shorter chains or alternative anions might help.
Taking the Next Step
People in labs—myself included—have seen firsthand how the right solvent changes an experiment. Chemical structure deeply influences physical and environmental properties. As understanding grows, solutions will follow. Responsible handling, ongoing research, and clear communication keep these innovative materials safe and useful for everyone.
Why Storage Matters
Some chemicals look harmless on the outside — they don’t fizz or burn your skin. That’s often the case with 1-Pentyl-3-Methylimidazolium Iodide. It doesn’t act up unless the environment is wrong. Freshness, safety, lifespan, and reliability all trace back to one thing: how you store it.
Learning From Hands-On Lab Work
Time in the lab teaches a lot about the quirks of ionic liquids. Shelves crammed with new bottles teach lessons about heat, light, and moisture faster than any textbook ever could. On multiple occasions, a bottle left under a sunny window or near a radiator changed appearance within days. It’s humbling. If you cut corners with storage, you waste money and raise risks.
Moisture Remains the Main Enemy
1-Pentyl-3-Methylimidazolium Iodide loves to pull water out of the air. Exposure to humidity turns a dry, stable salt into a sticky mess over time. You start with a powder—weeks later, you come back to a clump, and analysis shows it’s no longer trustworthy. Moisture wrecks purity and changes how it interacts with other chemicals. Once in that state, it’s hard to fix.
Heat and Light Are No Friends, Either
Consistent temperatures mean fewer surprises. Set up the storage zone in a cool, dry spot—think 2–8°C for best results. That’s the typical range for chemical refrigerators, not freezers. Going lower sometimes causes condensation as you go in and out, so the fridge route strikes a healthy balance.
As for light, too much of it encourages slow, sneaky chemical changes. A clear glass jar on an open lab shelf might look good, but after a season, the color deepens, particles form, and the bottle doesn’t pass quality control. Keep it in the dark: a brown glass bottle, an opaque container, or a closed cabinet. That single step prevents many long-term frustrations.
Sealing the Deal
Proper storage always starts with a real seal. Screw tops or custom caps keep out the air and slow leaks, nothing fancy needed. Silica gel packets or molecular sieves keep humidity low inside the bottle. At every professional lab I’ve seen, splitting stocks into small, single-use containers cuts down on waste. Every time the main bottle opens, it risks picking up airborne moisture or dust.
Labels and Regular Checks Prevent Headaches
A clear, waterproof label with the full chemical name and date of opening pays for itself in peace of mind. Checking each bottle every few weeks for color changes, clumps, or crystals tells you if your storage plan is working. Lot numbers and storage notes written right on the label remove guesswork if something turns out wrong downstream. Clean, practical habits like these make the difference between a smooth process and a ruined batch.
What Good Storage Brings
Handling special chemicals like this acts as a crash course in responsibility. Remembering a ruined vial and the scramble for a replacement orders new habits into your workflow. Spend a few more seconds on sealing, checking, and labeling to save hours rerunning experiments or replacing expensive stock. Good storage for 1-Pentyl-3-Methylimidazolium Iodide is about protecting both the science and the people around it.
Role in Pharmaceutical Development
Pharmaceutical labs look for consistent results and safe performance from every compound. This compound brings both to drug formulations. In research, scientists use it to build tablet coatings that withstand rough handling on production lines and shipping trucks. One reason manufacturers lean on this substance: it balances stability and solubility, so medicine holds up on the shelf but breaks down as needed inside the body. That balance matters because the difference between an effective treatment and a wasted dose can come down to the chemistry used behind the scenes.
Drug companies also count on this compound during the early testing phase when researchers watch for side effects or interactions. A well-designed formulation can help spot problems early. It supports drug delivery systems—such as capsules—and keeps ingredients dispersed evenly throughout a batch, which means fewer rejected batches and less lost investment.
Use in Food Technology
Grocery shelves stock products that last longer and taste fresher due in part to advances like this compound. Food scientists often choose it for its safety record and its ability to keep powders from clumping—nobody wants lumpy sports drinks or instant coffee. The compound can change the texture in processed foods like soups or desserts, helping products stay consistent from the factory to the family table.
My experience working alongside quality assurance teams taught me how tight regulations shape every step in the food industry. Ingredients must meet clear standards, and this compound’s long track record of safety helps production teams pass inspections while keeping costs under control.
Importance in Analytical Research
Lab technicians tracking contaminants or testing new products lean on this compound for clarity in their data. It often serves as a reliable standard or medium for analysis. Its purity and predictable behavior let researchers compare results from one experiment to the next. Even in advanced work like chromatography or spectroscopy, a stable compound gives confidence that findings make sense. Getting accurate numbers—whether for medicine, water safety, or industrial chemicals—can mean safer communities and sharper innovation.
Industrial Manufacturing and Materials Science
On the manufacturing floor, consistency is king. This compound shows up as a process aid, making sure machines run smoothly and finished products meet specifications. In plastics, rubbers, or even ceramics, it acts as a binder or filler to strengthen materials or reduce production costs. Factories have little patience for surprises; a reliable chemical ingredient keeps downtime low and quality high.
Materials scientists working to push boundaries in electronics, coatings, or structural components use this compound for its precise physical properties. For example, it can tweak the flow of a liquid or toughen a final product that needs to handle stress and heat.
Looking Ahead
Demand grows for chemicals with both proven benefits and responsible sourcing. Companies invest in transparent supply chains, regular safety reviews, and technologies that use less energy or create less waste. Increasing accountability in both research and industry makes a real difference in how innovations reach the market and serve society.