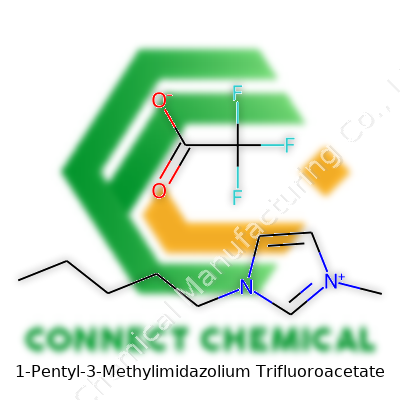
1-Pentyl-3-Methylimidazolium Trifluoroacetate: Insightful Commentary
Historical Development
Researchers started experimenting with imidazolium-based ionic liquids several decades ago. In the early years, most efforts focused on figuring out stable, liquid-phase salts that wouldn’t fall apart in humid air. Once chemists synthesized a whole range, it became clear some molecules, such as 1-pentyl-3-methylimidazolium trifluoroacetate, could take center stage for next-generation solvents. In university labs during the late 1990s, students like me kept bumping into classic imidazolium compounds in green chemistry readings. It wasn’t just about making “greener” solvents, but a real push to break away from stubborn, volatile organic compounds.
Product Overview
1-Pentyl-3-Methylimidazolium Trifluoroacetate brings together a long pentyl chain fixed to the imidazole ring, all balanced with a trifluoroacetate anion. You see bottles labeled with a viscous liquid that resists easy evaporation. The molecular design keeps it liquid at room temperature, one of the key goals when getting away from old-fashioned, flammable solvents.
Physical & Chemical Properties
In the lab, this compound usually appears colorless to pale yellow. Its melting point sits far below ambient, and the boiling point doesn’t really matter, since decomposition kicks in first. You’ll find it dense and sticky, with a faint, sharp odor—usually from the trifluoro groups. Unlike water or alcohol, its surface tension stays high. The ionic nature means almost no vapor pressure, which lets scientists work safely in open air without filling the room with fumes. Most ionic liquids resist ignition, and 1-pentyl-3-methylimidazolium trifluoroacetate is no exception, giving users an extra margin of safety.
Technical Specifications & Labeling
Product containers must bear the chemical name, CAS number, hazard symbols, and batch information for traceability. Many suppliers print QR codes on drums for digital tracking. Every label includes purity—often above 97%—plus recommended storage in dry, airtight bottles. Handling instructions warn about possible corrosion from trifluoroacetate, so standard lab gloves and goggles stay close at hand.
Preparation Method
Manufacturers often start with 1-methylimidazole and react it with pentyl halide to build the imidazolium core. The crude intermediate meets up with trifluoroacetic acid or trifluoroacetate salts in a metathesis step. After washing out extra reagents and drying under vacuum, the result is a ready-to-use ionic liquid. Sticking with low temperatures during synthesis prevents ugly byproducts. I’ve seen bench-scale setups do this in a morning, although scaling for industry needs bigger reactors and better temperature control.
Chemical Reactions & Modifications
This compound’s imidazolium ring allows easy substitution. That offers chemists the freedom to tweak cation properties or boost compatibility with other ionic liquids. The trifluoroacetate side can hydrolyze if water hovers nearby, sometimes releasing corrosive acids—an important risk to remember in moisture-prone labs. More advanced work uses 1-pentyl-3-methylimidazolium trifluoroacetate as a base to form functionalized ionic liquids through further alkylation or even polymer grafting, shifting its behavior from simple solvent to interactive reaction medium.
Synonyms & Product Names
You may spot this molecule listed as [PMIM][TFA], PMIM trifluoroacetate, or 1-pentyl-3-methylimidazolium trifluoroacetate across catalogs. Chemical retailers used to rename it by cation and anion initials, but standardization now favors the more detailed IUPAC version. Every lab book I’ve kept marks samples with at least two names, to prevent confusion when tracking inventory.
Safety & Operational Standards
Improper storage poses hazards. If moisture sneaks in, acid forms. Ventilated fume hoods curb accidental exposure, while sealed glass vials sit inside desiccators. GHS labeling applies here—users must understand pictograms for corrosivity and irritancy. Though less flammable than other solvents, it remains wise to keep fire extinguishers and spill kits within reach. Standard protocols mandate yearly safety training, based on chemical risk, and I’ve walked new interns through MSDS sheets more times than I can count. Long sleeves and splashproof goggles create a straightforward barrier against accidental contact.
Application Area
Industries reach for 1-pentyl-3-methylimidazolium trifluoroacetate for biomass processing, electrochemistry, analytical separations, and as a non-volatile reaction solvent in catalysis. Pharmaceutical companies experiment with it to solubilize difficult drug candidates, sidestepping old toxic solvents. Environmental researchers dissolve lignocellulose in it to unlock valuable sugars. In the energy field, it acts as a stable, ion-conducting medium for sensors or batteries. My own work touched solvents like this for green polymerization reactions that would struggle with more traditional setups.
Research & Development
Academic labs keep discovering new uses for ionic liquids. Some focus on its role in carbon capture, where its resistance to evaporation offers clear sustainability gains. Others look at using it to solvate complex proteins, opening doors in protein engineering and drug design. Scientists write up reaction conditions involving this compound, reporting everything from improved yield to milder working temperatures. Cross-institutional journals debate impurities, synthesis economics, and possible scale-up bottlenecks. Tech transfer offices work with industry to patent optimized protocols, so lab results make it into pilot plants.
Toxicity Research
Toxicological evaluations highlight important findings. Long-term studies in mammals show low acute oral toxicity for the parent compound, but its breakdown products demand caution, especially for lab animals sensitive to strong acids. Cell culture work tracks cytotoxicity at high concentrations, giving drug developers a baseline for safe use. Regular tests for bioaccumulation result in low risk compared to persistent industrial solvents. Still, regulatory bodies call for caution until more chronic exposure data surfaces. I’ve always made a point to keep spills off my skin and clean up right away, following recommendations that err on the side of prudence.
Future Prospects
Ionic liquids face a promising road ahead. With regulatory pressure squeezing older, volatile organics, researchers expect 1-pentyl-3-methylimidazolium trifluoroacetate and its cousins to move deeper into sustainable chemistry. As synthesis costs drop, use in biofuel refining, specialty batteries, and green separation processes will become everyday practice. Improvements in preparation technologies aim to cut down on hazardous byproducts, supporting environmental goals. Rigorous safety research marches forward, aiming to fill the remaining gaps in chronic toxicity knowledge, so both academic and industrial users can make confident, informed choices.
A New Tool for Solving Old Problems in Chemistry
Every few years in chemistry, a compound shows up that gives researchers a shot at breaking away from outdated, polluting methods. 1-Pentyl-3-Methylimidazolium Trifluoroacetate is one of those ionic liquids turning heads in both research labs and new-era manufacturing. Most folks outside the chemical sciences never see it, but its role runs deep where safer, more sustainable processes matter.
Making Biomass Useful
After working in a university lab, I remember how tough it got trying to break down raw plant material for clean energy or plastics. The old routes used acids and high temperatures, leaving waste and extra costs behind. Here’s where this ionic liquid flips the script: it can dissolve cellulose—one of the toughest natural materials—without those harsh conditions. Scientists use it to turn sawdust or straw into sugars, which serve as the base for biofuel or biodegradable plastics. No need for fossil fuels and strong acids, just a clever solvent and some time. That change means cleaner production, lower hazard levels, and new doors opening for green chemistry.
Slimming Down Industrial Waste
Chemical manufacturing churns out tons of solvent waste. Most of these are volatile and tough on the lungs, soil, and waterways. Companies look for alternatives that help them cut toxic emissions after several run-ins with fines and unhappy neighbors. I watched a colleague test ionic liquids—including 1-Pentyl-3-Methylimidazolium Trifluoroacetate—for tasks like separating chemicals and extracting valuable materials from mixtures. This stuff hardly evaporates and handles tasks at room temperature, bringing down both pollution and energy bills. For factories chasing regulatory compliance and cleaner images, that means more business and less cleanup.
Boosting Catalysis and Green Upgrades
One area where chemical progress never stops is catalysis—the art of speeding up chemical reactions. Many catalysts used today need supports and solvents that clutter the process or end up in the environment. Ionic liquids step up as the goldilocks zone: they dissolve nearly anything, offer a gentle environment that stabilizes fragile catalysts, and allow for recycling after the reaction. 1-Pentyl-3-Methylimidazolium Trifluoroacetate can enhance the yield of reactions that would otherwise falter, pushing things along with fewer toxic byproducts. Industrial chemists eager to cut their carbon footprints and improve efficiency see real value here.
Room for Improvement
Some folks raise concerns about the production cost and end-of-life impact of these newer solvents. No single fix covers every scenario. Researchers start with one bottleneck—such as cellulose processing or metal extraction—then adapt the molecule to fit after seeing how it works in practice. More life-cycle studies and larger pilot tests help clarify which applications justify the investment and which need tweaks. Everyday experience in the lab shows that real, useful change in chemical processes depends on continuous testing, transparency, and a willingness to rethink decades-old habits.
Moving Forward with Purpose
Picking the right solvent goes beyond lab performance and hits on practical issues like worker safety, environmental protection, and long-term cost. As academic and industrial teams keep growing their toolbox, 1-Pentyl-3-Methylimidazolium Trifluoroacetate’s place grows in specialty fields like green manufacturing, advanced materials, and biotechnology. The work gets shaped not just by technical progress, but by the shared drive to leave processes—and the world—in better shape than we found them.
What Drives Stability in Ionic Liquids?
Some chemicals fall apart quickly, others hold together for years. Ionic liquids, those salts in liquid form at room temperature, have drawn attention for their unique properties, from their low volatility to their ability to dissolve all sorts of stuff, even cellulose. Stability in these substances hinges on more than just lab synthesis. Their structure, moisture in the environment, impurities, light, and temperature all play a role.
In my own research years ago, I worked with 1-butyl-3-methylimidazolium hexafluorophosphate—a popular ionic liquid. We capped our bottles tight, stored them cool, and kept out water as much as possible. Even then, small leaks or careless handling sometimes turned clear fluids foggy or yellowish within months. The culprit? Hydrolysis and slow breakdown from humidity or accidental exposure to acids. This kind of thing is always lurking, not just in high-tech labs but in storerooms and shipping containers wherever these chemicals travel.
Why It Matters: Safety, Cost, and Efficiency
If an ionic liquid decays, its properties shift. Its melting point can climb. Color changes warn of side reactions that steal away performance. Once, I saw a project grind to a halt after a shipment sat in a sunlit warehouse—the liquid decomposed, the customer lost a week, and thousands in research went to waste. In business, repeated failures like this slash trust and balloon costs.
People in the field know that shelf life is not just a box to tick for regulators. Stability is the difference between a chemical winning over a tough industrial process or getting dropped from the lineup. Data from the Royal Society of Chemistry points out that some ionic liquids, such as those based on imidazolium with PF6-, can go six months to two years when stored right but might start losing strength if moisture creeps in. Phosphonium-based liquids usually fare better, owing to their robust cation structure.
Ignoring these facts means risking product recalls, environmental leaks, and equipment damage. End users—from battery makers to solvent engineers—count on advertised properties. No one wants to gamble on a liquid that decomposes overnight because no one asked the simple question: how long will this last, and what eats away at it?
Raising Reliability: Practical Steps Forward
Factory settings and shipping lines rarely match the gentle, dry air of research labs. Controlling shelf life starts with tight manufacturing control. Each batch needs robust quality checks—water content, acid scavengers, particle screening—before it moves down the line. My best results came with desiccant-packed storage jars, low temperatures (5–10 °C worked well for most), and amber glass to keep away sunlight, especially for photo-sensitive variants.
Suppliers must document and share real-time aging tests under various temperatures and humidity. That’s what makes a difference for end users picking between brands. Some companies use digital tracking for inventory, logging every vial’s history and condition—no more guesswork about whether a batch has sat through last summer’s warehouse heatwave.
Transparency projects from groups like the American Chemical Society encourage chemical vendors to deliver standardized shelf-life data instead of vague manufacturer estimates. This kind of open reporting helps every link in the supply chain, from chemist to carrier.
Ionic liquids hold promise for greener industry and smarter chemistry. Ensuring accuracy about their shelf life and chemical stability builds the trust and safety the world needs as these specialty fluids move into real-world applications.
Understanding What We’re Dealing With
1-Pentyl-3-Methylimidazolium Trifluoroacetate belongs to a family of ionic liquids that’s finding more use across chemical labs and industry. I’ve seen my share of bottles with complicated names and complicated risks. Not every chemical wants the same home on your storage shelf. Overlooking proper storage leads to all sorts of trouble—from breakdown and lost money to real danger.
Safe Storage Keeps You Ahead
I remember helping a research group store a small bottle of this stuff a few years back. We learned early on it reacts badly with moisture and doesn’t like sunlight. Anyone who’s seen what water does to ionic liquids knows the results: cloudiness, byproducts, and ruined experiments. That’s not just a waste; you also risk creating something toxic or corrosive nobody wants to clean up.
Glass, high-density polyethylene, or fluoropolymer bottles provide a strong defense. These types of containers mean you’re less likely to run into surprises—the sort that dissolve plastics or break seals. Keeping things tightly closed always reduces the risk of leaks or contamination. I once made the mistake of leaving a cap a little loose on a bottle of another ionic liquid, and returning to a sticky, useless mess made a permanent impression.
Keep Cool, Keep Dark, Keep Dry
Most labs aren’t temperature-controlled like a pharmaceutical warehouse, but you can pick the spot with steady, cool air. Setting the bottle on a shelf away from radiators, hot plates, and windows makes a big difference. Heat slowly degrades many ionic liquids, and this one’s no exception.
Bright light, especially sunlight, speeds up breakdown. Chemical bonds might not snap all at once, but over weeks or months, the bottle’s contents won’t work as intended. I always stash sensitive materials in a cupboard or opaque bin. If you add a silica gel packet or another desiccant, you cut back on unwanted moisture sneaking in each time the lid opens.
Labeling and Inventory Control Matter
Too many labs store chemicals with faded stickers or incomplete information. Writing the full chemical name, concentration, batch number, and the date you opened the bottle pays off. No more guessing ten months from now about what the substance is or whether it’s still safe to use.
Regular checks cut down on unpleasant surprises. During my grad school days, a periodic cleanup revealed way too many forgotten bottles with questionable contents. Eliminating old or questionable stock keeps everyone safer, especially in a shared space where not everyone knows what’s in every cupboard.
Plan for Spills and Accidents
Clean workspace habits come as much from experience as from rules. Keeping absorbent pads, gloves, and goggles ready beats scrambling if something spills. If an accident happens, nobody wants to hunt for cleanup supplies. Spill kits specifically rated for ionic liquids handle this class of chemicals better than general-purpose ones.
Wrap-Up: A Practical Routine Works Best
Take the time for proper storage, labeling, and periodic checks. Ask questions. Don’t just follow the instructions from manufacturers—combine them with real-world experience. Informed choices save resources, protect health, and keep chemistry moving forward.
Getting to the Bottom of Safety Concerns
Everyday folks see warning labels on packages and often overlook what they mean. The reality is, loads of products in homes, schools, and workplaces come with hidden risks. Years back, I swapped out a basic cleaning spray under the kitchen sink for a greener alternative, only to realize the original bottle carried a small skull symbol. That woke me up. Chemicals, batteries, paints, and even certain glues can cause harm if handled carelessly or tossed out with normal trash.
Take batteries, for instance. Toss one in a campfire, and it might explode. A kid once brought an old lithium coin battery to school for show-and-tell; a teacher spotted it before the class started, saving potential panic and injury. Lithium batteries swell, leak, or catch fire if crushed or overheated. Local news in 2023 covered several house fires traced back to improper disposal of such batteries. Safety experts and firefighters keep repeating: isolate used batteries, store them in a non-metal container, and deliver to collection centers.
Stories from the Shop and the Shed
I remember painting my front porch and ignoring those pungent solvent warnings. Two hours in, my head pounded, and my hands tingled. Paint thinners and strong cleaners release fumes that displace the air in poorly ventilated rooms. People fail to open windows or skip the dust mask, thinking it’s overkill. Hospitals record thousands of accidental poisonings every year—many involving everyday products misused at home.
Garage work brings its own problems. My neighbor stored muriatic acid in an old soda bottle. His son took a swig, thinking it was lemon-lime. After a frantic trip to the ER, the family keeps that stuff locked up, clearly labeled, away from food or drink. Lessons like this stick with you. Simple steps—labels, lock-ups, gloves, and masks—make a huge difference.
Why Proper Handling Matters
Ignoring safety recommendations is never worth the risk. Thousands of workplace injuries come down to skipping procedure when dealing with solvents, acids, or heavy-duty cleaners. The Occupational Safety and Health Administration (OSHA) updates rules based on growing lists of incidents. Store employees, teachers, janitors, and busy parents might think a little shortcut won’t hurt, until it does.
Ingredient lists reveal what most people miss. Anything with “corrosive,” “flammable,” or “toxic” on its packaging deserves respect. The Environmental Protection Agency keeps a searchable database for those curious about specific products. Facts don’t lie: accidental poisonings, fires, and burns happen every week across the country because folks didn’t read or skipped precautions.
Building Safer Habits at Home and Work
Solutions exist and aren’t expensive. Keep products in their original packaging. Store them out of reach of children and pets. Seek safer alternatives when possible—many brands offer low-toxicity options now. Look for third-party certifications and ratings on household goods. Community drop-off days for hazardous waste make disposal simple.
Education counts. Share stories and facts with neighbors or coworkers. An informed family or team looks out for each other. Smart habits spread when people see their value in real terms. By taking warnings seriously, we stop accidents before they start, protect our health, and look after the environment we all share.
Purity in the Context of Ionic Liquids
1-Pentyl-3-Methylimidazolium Trifluoroacetate doesn’t land on a chemist’s shelf by accident. Labs often look for this ionic liquid because it acts as a solvent, catalyst, or extracting agent in everything from cellulose processing to battery research. Not all bottles of this chemical sit at the same level of purity, and that single factor shapes both what a researcher can do AND what results emerge at the end of a process.
Industry Purity Benchmarks
Researchers usually find this compound listed in three broad purity levels. Technical grade varieties hit somewhere between 95% and 98%. These go into early-stage method development or screening experiments. After that, labs often reach for 99% purity or higher—sometimes labeled as analytical or research grade. For highly sensitive work, such as NMR spectroscopy or organic electronics, some providers produce lots with purity above 99.5%. Detailed certificates of analysis might even report to two decimal places (for example, 99.86%) when trace contaminants can skew a project’s outcome.
Real-World Impact of Impurities
Every chemist who’s handled ionic liquids has a story about how a few stray percentages change everything. Water content, for starters, can trigger side reactions. Simple organic residues left from manufacturing sometimes scaffold new reactivity inside carefully controlled reactions. Even a few tenths of a percent of inorganic salts will corrode sensitive electrodes in electrochemical devices. I’ve seen PhD students hunt for contamination sources, only to find a “95% pure” label hiding a troublesome ingredient not listed on a data sheet.
Testing and Vendor Transparency
Not all purity claims actually hold up. Manufacturers often screen with NMR, IR, and mass spectrometry, but some only test for expected byproducts—missing the unforeseen. A lab working on something new should ask for full certificates, including details on residual solvents, halides, or water content (KF titration numbers often matter here). A supplier willing to hand over full analytical traces signals there’s nothing to hide. Those who dodge questions probably don’t serve you well during tricky validation work.
Solutions for the Purity Dilemma
Purifying in-house offers one path. For certain needs, distillation under reduced pressure or readily available drying agents will scrub out most water or volatile organics. More stubborn contaminants usually require column techniques, which not every lab wants to set up for ionic liquids. The best results typically follow a system of joint verification—comparing supplier’s figures with your own testing before starting a high-value experiment. Cutting corners to save a few bucks at the start often burns through weeks of troubleshooting down the road.
Why the Right Purity Changes Everything
A bottle labeled “99% pure” sometimes represents the single variable between a published result and a failed replication. Multi-million-dollar industries—biofuel producers, pharmaceutical labs, green chemistry startups—rely on robust, repeatable outcomes. Small labs risk all their funding if a single impurity goes unnoticed. Trust builds in the transparent back-and-forth between producers and the users grinding through real samples in real environments. Getting the right purity isn’t just about compliance but creating a foundation researchers and manufacturers can believe in, batch after batch.