1-Propyl-2,3-Dimethylimidazolium Hexafluorophosphate: A Deep Dive
Historical Development
Talking about ionic liquids, chemists in the late 20th century started seeing these versatile compounds as real alternatives to volatile organic solvents. 1-Propyl-2,3-dimethylimidazolium hexafluorophosphate belongs to this class. In the 1990s, the rising interest in green chemistry helped drive research on these salts. Industrial labs began shifting attention to ionic liquids because of their negligible vapor pressure and stability, and among these, 1-Propyl-2,3-dimethylimidazolium hexafluorophosphate made a mark for its strong cation and robust anion pairing, making it suitable for various demanding applications. Its onboarding into research environments gave chemists the edge to explore new electrochemical processes, recycling techniques, and cleaner synthesis routes. As demand for cleaner laboratory practices grew, usage of such chemicals didn’t just stay in academic circles; they moved into pilot plants and tech firms focused on battery tech and specialty synthesis.
Product Overview
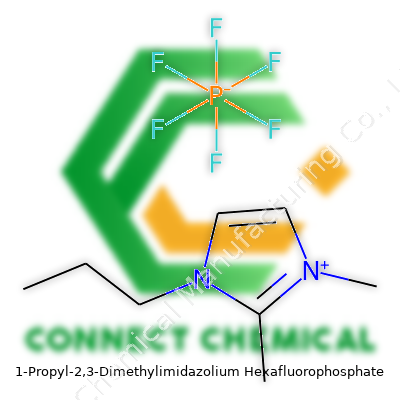
1-Propyl-2,3-dimethylimidazolium hexafluorophosphate falls into the imidazolium-based ionic liquids segment. Its structure sports a propyl side chain and two methyls attached to an imidazole core, carrying a hexafluorophosphate counterion. Chemists and engineers rely on this compound in processes needing a stable, hydrophobic, and non-volatile medium. From my lab days, working with this compound meant enjoying fewer headaches about evaporative losses and easier containment. Laboratories handling sensitive electrochemical systems frequently turn to it, particularly in areas where a non-aqueous, non-coordinating solvent brings out better selectivity or stability in tricky reactions.
Physical & Chemical Properties
This ionic liquid stands out for its colorless to light yellow liquid appearance at room temperature. Thanks to its structure, it won’t boil away under standard lab conditions. Densities float just above 1.3 g/cm³, and its melting point often sits well below standard room temperatures—past experiments clocked a glass transition temperature below -30°C. Chemically, the hexafluorophosphate anion resists hydrolysis and won’t corrode steel or glassware under normal conditions. Its viscosity ranges higher than traditional solvents, which needs consideration during process design, especially when stirring or pumping. The thermal stability runs up past 200°C before decomposition, letting users crank up temperatures for special syntheses without worrying about breakdown. Conductivity numbers, aided by the imidazolium framework, serve well in electrochemistry, and the low volatility takes away explosion hazards present in classic organic solvents.
Technical Specifications & Labeling
Vendors typically list 1-propyl-2,3-dimethylimidazolium hexafluorophosphate using purity bands above 98%. Water content tells a lot about how rigorously the batch got dried, with some researchers requiring levels below 200 ppm. Labels often show both short and expanded chemical names, sometimes shortened to the acronym [PrDMIM][PF6], CAS Number 671973-55-8, and relevant batch numbers for traceability. Given its use in research, most bottles include hazard symbols for irritants and environmental warnings based on the hexafluorophosphate ion. Lot-specific analytical data, including NMR and ion chromatography, give end-users a sense of purity and possible impurities. Still, scrutiny on trace halides and related ions dominates quality assurance, spurred by years of experience with contamination derailing sensitive experiments.
Preparation Method
Crafting this ionic liquid follows a standard protocol. A starting imidazole undergoes alkylation reactions, usually using propyl halides and methylating agents in aprotic conditions. Stepwise alkylation tweaks temperature and reagent ratios to maximize yield and avoid unwanted side products. After building up the cation, introducing hexafluorophosphate comes next—most often by metathesis from sodium or potassium hexafluorophosphate. The crux lies in thorough washing and drying of the resulting liquid, scrubbing away halide contaminants with repeated aqueous extraction. Oils left behind show up pristine after drying under high vacuum, ready for further purification if research requires sub-ppm water levels. Handling the product demands proficiency; hexafluorophosphate sources demand gloves, a fume hood, and a real nose for spotting HF formation.
Chemical Reactions & Modifications
Once in hand, this liquid lends itself to derivatization. Chemists swap the anion for others—like tetrafluoroborate or bis(trifluoromethylsulfonyl)imide—changing properties such as viscosity and hydrophobicity. Cation tweaks, achieved by swapping out the propyl or methyl groups, shift polarity and transport properties. In practical terms, these modifications tailor the solvent space for catalyst stabilization, separation science, and energy storage tasks. Electrochemists exploit its stable electrochemical window for both oxidative and reductive research without the interference seen from more reactive solvents. With this ionic liquid as the base, changing its chemical character shapes the future of catalysis, extraction, and green process engineering—something I’ve watched grow from the benches of small-scale testing to kilo-lab pre-commercial demos.
Synonyms & Product Names
Trade literature and catalogs feature this compound under synonyms like [PrDMIM][PF6], 1-propyl-2,3-dimethylimidazolium hexafluorophosphate, and less commonly as C8H16F6N2P when listed by formula. Some specialty chemical suppliers shorten it to PrDMIM-HFP, and you might see versions carrying their manufacturer prefix, such as “Sigma PrDMIMPF6.” Its chemical identifiers (CAS, EC number) tag it across safety data sheets, avoiding confusion in international shipping or regulatory databases. I’ve seen mistakes happen when only “imidazolium hexafluorophosphate” appears in an order—clear labeling saves both time and accidents.
Safety & Operational Standards
Workplace safety can’t ever take a back seat with this class of compounds. Despite the non-volatile nature, hexafluorophosphate salts may slowly hydrolyze, generating trace hydrofluoric acid, a real concern for skin and eye protection. Labs and factories keep HF antidote gel nearby and insist on nitrile gloves, long sleeves, and goggles for all handling. Spills stay rare, thanks to the liquid’s low volatility, but cleanup uses calcium gluconate for neutralization and disposal in fluorine-compatible waste streams. I remember training new staff—underestimating hexafluorophosphate dangers leads to lax standards, and even a slip in cleanup can cause issues down the line. Storage in sealed plastic or Teflon containers, away from ambient moisture, preserves quality and reduces risks linked to decomposition.
Application Area
This ionic liquid finds homes in diverse research and industry spaces. Battery development teams favor it for high-voltage electrolyte blends, drawing on its wide electrochemical window and resistance to electrolysis. In catalysis, 1-propyl-2,3-dimethylimidazolium hexafluorophosphate offers a solvation environment for metal complexes and enzymes, helping drive reactions cleaner than conventional solvents. Separation processes, especially tricky extractions involving rare earths or organometallics, gain selectivity and efficiency with its low miscibility with water. Supercapacitor engineers, fuel cell developers, and materials labs all touch this compound at some stage as they search for robust, environmentally friendlier process materials. Despite its cost, benefits in performance, process safety, and environmental regulation compliance keep it relevant.
Research & Development
Academic and industrial researchers devote substantial effort to making these liquids both cleaner and more effective. Development targets include refining synthesis routes, reclaiming used liquids, and developing hybrid solvents tuned for specific reactions. Every round of testing spins off new data on conductivity matrixes, viscosity-temperature profiles, and compatibility with advanced materials. Collaborations between chemists and engineers seek to unlock full recyclability; after all, the upper-tier ionic liquids command premium prices. Green chemistry initiatives drive academic labs to share open-source purification protocols and sustainable waste management strategies, building on lessons learned from both small- and large-scale usage.
Toxicity Research
Safety researchers dig deep into the biological impact of both the cation and the PF6 anion. Lab trials show low acute oral and dermal toxicity for the cation, though chronic effects or high-dose exposures get less coverage. Hexafluorophosphate’s stability means limited release in standard operation, yet degradation products—like fluoride ions—raise health and ecological flags. Studies on fish and invertebrates show moderate environmental risks if ionic liquids reach water streams, while soil persistence analysis suggests these chemicals won’t degrade quickly. For waste streams, this means specialty incineration or chemical neutralization remains best. Risk assessments encourage tracking every disposal, aiming to stay ahead of regulatory changes. I learned early to track every gram used and disposed, not only from a safety perspective but to keep future lab audits easy and transparent.
Future Prospects
As the chemical industry leans into sustainable production, 1-propyl-2,3-dimethylimidazolium hexafluorophosphate stands to either grow in relevance or hand off its role to even greener, less fluorinated successors. Research pushes for designing ionic liquids with minimal environmental footprints, drawing on lifecycle analysis tools, and maximizing recyclability. There’s momentum behind blending in bio-based cations and swapping out PF6 for less persistent or less toxic anions. The march toward decarbonization and stricter chemical regulations in the EU and Asia means future ionic liquids must clear higher bars for safety, cost-effectiveness, and environmental compatibility. My own take, watching policy and science meet, says these compounds could anchor new tech revolutions from battery innovation to selective extraction—especially if production routes shed complexity, lower risk, and close the loop on waste streams.
Where Science Meets Daily Application
My chemistry background led me to cross paths with a substance called 1-Propyl-2,3-Dimethylimidazolium Hexafluorophosphate, a name that feels straight out of a tongue-twister competition. Underneath the technical name, this compound grabs the attention of chemical engineers and synthetic chemists looking for ways to make processes cleaner, faster, and safer. The world knows a lot of buzzwords about “green chemistry” and “ionic liquids,” but this particular imidazolium salt actually deserves some credit for contributing to real-world solutions.
Driving Cleaner Chemistry
Ionic liquids tend to show up in labs when someone wants to separate things or create new stuff—batteries, pharmaceuticals, specialty materials. What makes 1-Propyl-2,3-Dimethylimidazolium Hexafluorophosphate special isn’t its unspellable name, but its knack for dissolving a wide range of substances and staying stable under conditions that few other liquids can handle. I’ve seen it used as a non-volatile solvent, replacing traditional organic solvents that give off harmful fumes, especially in catalysis and electrochemistry. When chemists aim for less toxic waste and fewer headaches from working with flammable solvents, compounds like this one step up.
Boosting Battery and Solar Tech
In tech circles, batteries and solar panels draw lots of hype, but their inner workings don’t improve without materials that push the boundaries. I’ve read journal studies showing how imidazolium-based ionic liquids help lithium-ion batteries last longer or stay safer at higher temperatures. This compound doesn’t burn or evaporate easily, so battery manufacturers keep an eye on it to slow down failures and prolong cycles. Some labs even test it in dye-sensitized solar cells as an electrolyte. Between the green goals and the need for better energy storage, 1-Propyl-2,3-Dimethylimidazolium Hexafluorophosphate finds itself in prototypes aimed at making renewable energy competitive.
Cleaning Up Heavy Industry
Outside the lab, fields like metallurgy and mining need breakthroughs to deal with toxic sludge and metal recovery. Industries have started looking at this ionic liquid for extracting rare metals or recycling waste because it can hold onto metals like gold, platinum, or palladium without using high temperatures or strong acids. As someone who tracked wastewater cleanup for a summer project, I remember the headaches of trying to filter out valuable metals mixed in grime. Using this ionic liquid means fewer steps and less impact, though the cost and recycling of the solvent itself often turns into a sticking point. That’s a problem worth tackling with large-scale testing, which already started happening in Asia and parts of Europe.
Challenges and Paths Forward
No chemical is perfect. Questions remain about the long-term effects if ionic liquids seep out into the wider environment or get mishandled in manufacturing. Recent independent reports flagged concerns about the persistence of hexafluorophosphate salts in water, and some scientists argue for rigorous tracking from cradle to grave. I agree with those calling for better data—not just to find what’s possible, but to make sure these green solutions create fewer headaches than the old ways. Frequent communication across industries can drive safer scales of production and new recycling strategies.
From personal observation, the future of 1-Propyl-2,3-Dimethylimidazolium Hexafluorophosphate looks like a balancing act between promise and responsibility. Innovation matters just as much as oversight. This compound highlights that chemistry doesn’t just stay in flasks and journals, but shapes real progress, one tricky molecule at a time.
Getting Serious About Protective Gear
Working with chemicals means thinking about safety before anything else. I still remember my earliest days in the lab — before touching a single bottle, my supervisor pointed straight at the goggles, gloves, apron, and face shield on the shelf. Chemical burns and vapors don’t care if you’re careful only most of the time. Closed-toe shoes and fitted gear keep spills off your skin, and those safety goggles? They shield eyes from both splashes and unexpected reactions. Too many stories float around labs about careless folks landing themselves in the ER over forgetting gloves, or worse — choosing to “just be careful.”
Ventilation Stops Trouble Before It Starts
Pulling open the hood isn’t just about following rules. Strong fumes in a closed space can make you dizzy, cause breathing trouble, or wreck your sense of smell. Good airflow draws vapors out of your face and away before they soak in. Properly working fume hoods or exhaust fans take away the bulk of danger fast. At my last job, a coworker ignored a half-clogged vent, claiming it didn’t matter for only a few drops. He got a week’s worth of headaches and a stern lecture from the safety team. It pays to keep those filters clean and check air movement with a quick strip of paper every so often.
Read Labels Like Your Health Depends On It
That scribbled short name on a jar doesn't tell the whole story. Chemical labels and safety data sheets lay out everything — from explosive risks to what happens if you inhale dust or let it touch your skin. Knowing what you’re working with isn’t paranoia, it’s plain common sense. Once, I grabbed the wrong container from a crowded shelf and learned the hard way that not all acids burn at the same rate. Checking labels and cross-referencing SDS sheets for things like incompatibilities (water with acid? Not unless you want fireworks) keeps you out of the panic zone.
Storing Chemicals for Long-Term Safety
Strong acids, bases, and solvents shouldn’t sit wherever there’s an open shelf. Flammable liquids need a fire-safe cabinet. Acids belong away from anything reactive, especially ammonia or bleach. Chemical storage might sound like nitpicking, but keeping everything in the right spot avoids dangerous mix-ups and accidental reactions. At one workplace, a leaky bottle of bleach wound up too close to some ammonia. Cleanup crews suited up and evacuated half the building before things calmed down. Shelving plans and storage labels prevent those nightmares.
Spill Response: Fast and Calm
Nobody wants to deal with a chemical spill, but being prepared turns a bad day into a manageable one. Spill kits with neutralizing agents, absorbent pads, and protective equipment belong in arm’s reach. The first step is keeping calm and not trying to scoop liquid with bare hands. Alerting coworkers, airing out the space, and using the right cleanup products goes much farther than grabbing paper towels. Accidents happen, but knowing what to do saves time and avoids injury.
Training Never Ends
Most places run safety training once a year, but real safety comes from daily habits and honest conversations. Watching experienced colleagues handle tricky chemicals, asking questions even if they sound basic, and speaking up when something looks off creates safer spaces for everyone. The right attitude turns safety rules from an obligation into second nature. That alone can mean fewer close calls and less time spent in recovery rooms.
Understanding the Basics
Chemistry comes down to understanding connections and proportions. Every compound has a unique molecular formula that tells you which atoms show up and in what numbers. Consider water. We all know H₂O. That's two hydrogens and one oxygen. The formula unlocks everything about how a substance behaves, who it interacts with, and what it can do.
I’ve worked in labs where even a single misplaced number in a formula can throw off entire experiments. Getting the molecular formula right is about more than just numbers on paper. It's the starting line for understanding whether a compound reacts explosively with air, cures disease, or forms a sturdy plastic. Mistakes aren’t just embarrassing – they’re dangerous. Toxic substances or runaway reactions can result from getting the formula wrong. Accurate information keeps people safe.
The Numbers Behind the Name
The molar mass brings this information down to earth in practical ways. It represents the weight of one mole of a particular compound. Chemists reach for molar mass every single day – dissolving chemicals in water, balancing a chemical reaction, even deciding which containers to choose. A whole industry may depend on knowing exactly how much of a substance they need to order or ship. Waste and cost both come down when you have the right numbers.
Calcium chloride, CaCl₂, is more than just a snow-melting salt on my driveway each winter. Its molecular formula tells me it has one calcium and two chloride ions per molecule. The molar mass (approximately 110.98 grams per mole) guides companies in mixing solutions for labs, roads, or industry. Hospitals use precise doses in IV solutions. A mistake means either too little effect or, worse, patient harm.
Real-World Consequences
Pharmaceutical companies live and breathe valid molecular data. Imagine a drug with an unknown or misreported formula. People get sick, lawsuits pile up, trust evaporates. Even in agriculture, the wrong formula in a fertilizer can stall crop growth or poison a field. Quality rests on simple arithmetic: knowing what you’re working with, and how much. If you’re making something like aspirin (C₉H₈O₄), you want each batch as consistent as the last. No guessing games.
Opportunities for Better Practices
Transparency in chemical databases creates a safer world. Open-source projects and online repositories let students, researchers, and industry workers double-check facts. Still, plenty of compounds come with incomplete data or flat-out mistakes reported by out-of-date sources or dubious suppliers. If you’re in charge of procurement, that can get expensive or hazardous fast.
Classrooms and labs would do better by teaching not just rote memory, but ways to verify a formula and check the math on molar mass. I’ve seen people skip the steps because they trust a supplier’s label, and it’s always a risk. A second set of eyes, double-checking with a periodic table, often reveals mix-ups. Tech tools and apps that instantly calculate mass from a formula help reduce human error.
Looking Forward
Making molecular formulas and molar masses accessible doesn’t just simplify work for skilled chemists. It takes fear and guesswork out of science and industry. Publishing accurate information and cultivating habits of verification raise the bar for everyone, from high school students to chemical engineers in bustling plants worldwide.
Chemicals Like This Demand Respect
Lots of people get used to tossing solvents onto a shelf. That won’t work for 1-Propyl-2,3-Dimethylimidazolium Hexafluorophosphate. This salt, part of the ever-expanding world of ionic liquids, brings both promise and a set of headaches. It isn’t the sort of chemical you trust in a warm, humid space. Moisture sneaks up on ionic liquids, turning useful stuff into hazardous trouble fast.
Temperature and Moisture Control Aren’t Suggestions
Don’t assume room temperature means a good spot. This chemical acts temperamental with heat, so it belongs in a spot kept below 30°C. Experience has shown me corners of the lab get hotter than you expect, especially near windows or behind processors. We once stored an ionic liquid on top of a fridge thinking it was out of the way—it absorbed moisture from the air and lost integrity. A dry, air-conditioned store room with a stable temperature makes a big difference.
Dampness matters more than you think. Leave the lid loose, even for a few hours, and hydroscopic chemicals like this one suck up water quick. The result? Pure samples turn cloudy, ruining experiments. Lids need sealing, every time. Invest in desiccators or stock a reliable drying agent in your storage cabinet. Silica gel packets save more than paperwork—they help keep chemicals fit for use.
Material Compatibility and Labeling
Metal shelves sometimes corrode, especially if a chemical leaks. Non-metal containers like HDPE or amber glass don’t just resist corrosion—they stop stray UV rays and oxygen from picking apart your compound. I’ve seen clear bottles next to a window yellow out in six months. Shielding from light and air cuts down on waste.
No mystery containers, either. Clear, dated labeling with the full name, hazard details, and date opened stops mistakes. I’ve fumbled over unmarked vials and learned the hard way. Labels keep people safe, especially in busy labs where supplies change hands fast.
Keeping Hazards In Check
Hexafluorophosphate leeches out hydrofluoric acid when it breaks down. Even trace amounts of moisture or high temperatures make this more likely. Anyone who’s spilled a vial and smelled the harsh sting knows the risks for skin and lungs. That acid causes burns you don’t forget. Spill kits, gloves, and eye protection are not extras—make a habit of using them.
Ventilation matters. A sealed chemical storage cabinet with good air exchange slows the spread of vapors and keeps both air and people healthy. I’ve worked in tight stockrooms with no airflow and headaches come quick. Fresh air keeps labs safe and functional.
Safer Labs Start With Simple Habits
Following the safety data sheet is only a start—you build habits from years of handling tricky chemicals. Store everything with dry hands. Put away containers right after use. Inspect for leaks or discoloration at least once a week. These habits saved our lab thousands of dollars and more than one person from a trip to urgent care.
People focus on fancy extraction and analytical tools, but safe chemical handling works as the backbone of any lab. Taking storage seriously builds trust between labmates and keeps experiments running. Safety isn’t always a dramatic story; often, it’s a well-sealed container under the right light, in the right spot, every time.
Tackling Everyday Chemical Confusion
I remember my early days working in a shared lab, watching bottles of solvents line the shelves—acetone, toluene, isopropanol—each promising endless ways to blend and clean. Mixing them with certain products didn’t always go to plan. At best, the combination left a goopy mess. At worst, it bubbled, smoked, or ruined expensive glassware. Compatibility goes beyond reading a label. Beyond flashy marketing, daily lab safety and cost savings start with knowing what reacts with what.
What Actually Happens When You Mix Things
A product reacts with solvents based on its chemical structure. I’ve learned not to trust quick checklists. Let’s say you want to dissolve a polymer in a common reagent—acetone might make it clear, but methanol leaves it floating in chunks. Water often gets overlooked, but even a few drops can split an emulsion or kill a reaction. I once watched a colleague lose a day’s work adding a “safe” solvent to a resin, only to end up with a milky mess. No two products melt, split, or react quite the same way.
Manufacturers print certificates and guidelines, but few spell out what matters: purity, temperature, or what else has touched that flask. Research from chemical safety resources such as the National Institutes of Health and material safety data sheets points toward trial and observation. The devil lives in the details—impurities spark flames, moisture ruins a catalyst, and sunlight speeds up rot or discoloration. Open containers love to suck in air, making yesterday’s perfect mix useless today.
Getting the Facts Straight
A good technician never guesses. I rely on collaborative tools: Merck Index, PubChem, and ChemSpider offer compatibility charts, but it helps to double-check the chemical family, especially with new or specialty materials. One trusted source is Sigma-Aldrich’s solvent compatibility tables—they spell out which esters, chlorinated hydrocarbons, and alcohols safely blend with target compounds, and which ones don’t. Whenever flammables hit the bench, my go-to move is to check compatibility with both the solvent and the container itself. I’ve seen rubber degrade instantly in cyclohexane, and plastic get soft in DMSO.
Common Pitfalls and Smart Habits
Every field, from pharmaceuticals to art restoration, runs into mixing mishaps. Painters learn fast which thinners destroy synthetic pigments. Pharmacists keep an eye out for drug precipitates, especially when someone asks about odd combinations off the shelf. A polymer chemist reading an old forum post can spot a rookie mistake in seconds, because the rare stories—milky mixtures, unexpected precipitates, or heat spikes—say more than a hundred press releases.
The fix comes from blending experience with data. I’ve learned to start with small test batches. Label every sample, watch for unexpected color, settling, or heat. Ventilated hoods and gloves beat cleaning up a mess. A brief pause unlocks deeper understanding—ask a colleague, call a supplier, or even consult academic literature. Smart science means knowing every bottle’s quirks before risking a new mix.
Looking Ahead: Why Compatibility Still Matters
Thousands of products hit the market every year, promising speed and simplicity. Behind the scenes, chemists and technicians rely on shared experience, published research, and proper caution. Teaching new team members to test compatibility before they scale up saves money, headaches, and sometimes even lives. Too many shortcuts become expensive lessons. Not every product fits with every solvent, and the smartest labs always check and double-check before diving in.