1-Propyl-3-Methylimidazolium Dicyanamide: A Down-to-Earth Commentary
Historical Development
The story of 1-Propyl-3-Methylimidazolium Dicyanamide tracks with the evolution of ionic liquids. Not so long ago, chemists focused on traditional solvents—volatile, often dangerous to health and the environment. Then, in the late twentieth century, researchers saw possibilities in new salt-based liquids. Imidazolium-based ionic liquids stood out thanks to their stability and tuneable features. By the time 1-Propyl-3-Methylimidazolium Dicyanamide caught the eye of the chemical industry, academic labs were already running with the idea of “designer solvents.” Cations like 1-propyl-3-methylimidazolium had proven their flexibility, and dicyanamide anion offered intriguing reactivity—a marriage of properties that set the stage for wider use not only as a cleaner solvent, but as an active ingredient in synthesis and materials science. Through the steady accretion of thesis work, published articles, and conference talks, this particular ionic liquid took form as a reliable building block in green chemistry.
Product Overview
Seen in both bottles on lab shelves and in industrial tanks, 1-Propyl-3-Methylimidazolium Dicyanamide stands as a room-temperature ionic liquid. Most chemists recognize it simply as [C3mim][DCA]. It earned a reputation for being more than a one-trick pony, balancing inertness, low flammability, and a special solvation power that attracts both organic and inorganic chemists. Compared to volatile solvents like toluene or acetone, [C3mim][DCA] brings lower vapor pressure—helpful for folks working in stuffy or poorly ventilated spaces. It dissolves an impressive range of compounds, carrying out roles in electrochemistry, catalysis, extraction, and analytical work. People in research labs keep returning to it, whether purifying pharmaceuticals, running catalytic experiments, or seeking new ways to process metals.
Physical and Chemical Properties
Looking at its physical properties, [C3mim][DCA] typically appears as a clear-to-light yellow liquid around room temperature. Its melting point often falls below 20°C, sometimes closer to 0°C, which keeps it useful in both warm and chilly environments. With a density above water—between 1.05 and 1.2 g/cm³—this liquid doesn’t evaporate easily. Its moderate viscosity, usually less than other similar ionic liquids, makes stirring and pumping a lot more manageable. On the chemical side, the imidazolium cation resists oxidation under mild conditions, while the dicyanamide anion reacts with both nucleophiles and electrophiles. This combination provides wide compatibility with metals, organics, and gases, even letting users adjust polarity in multi-solvent mixes.
Technical Specifications and Labeling
Industry labeling asks for clarity, so most suppliers detail purity—often over 98%. They must include water content (many list under 0.5%) since moisture influences solubility and stability. Technical sheets also note the compound’s molecular weight (about 192.25 g/mol), viscosity, pH (neutral to slightly basic), and recommended storage temperatures (between 5°C and 30°C). Safety data demands full seriousness. Packaging often comes in sealed amber bottles to block UV, with clear hazard symbols for eye and skin irritation risks. MSDS sheets explain toxicity, reactivity, and required handling steps. This tight attention to labeling reflects regulations from OSHA, REACH, and GHS—details most users glance at often, especially with large-scale production or export in mind.
Preparation Method
Labs usually prepare [C3mim][DCA] by metathesis—switching out counterions between imidazolium halide and an alkali metal dicyanamide salt. Most chemists start by dissolving 1-propyl-3-methylimidazolium bromide in water, then mixing in sodium dicyanamide. The dicyanamide swaps places with bromide, forming [C3mim][DCA] in solution. Next, they extract the ionic liquid with an organic solvent, such as dichloromethane or ethyl acetate, to remove leftover sodium bromide. After separation, the organic layer is washed, dried (usually over magnesium sulfate or in vacuo), and filtered. Final purification steps—like vacuum distillation—clear up color and trace water, ensuring high purity. This process, while straightforward for small batches, gets trickier at scale, where full removal of halide and moisture means proper equipment and experienced hands.
Chemical Reactions and Modifications
1-Propyl-3-Methylimidazolium Dicyanamide doesn’t just sit inert in the bottle. The dicyanamide anion can coordinate with metal centers, supporting catalytic cycles or helping break up stubborn mineral lattices. Chemists working in organometallics prize it for these reasons. Besides complexation, the anion allows for post-synthetic modifications, like introducing new cations via ion exchange for different applications. The imidazolium ring, under controlled reaction, stands up to mild alkylation but shows sensitivity to strong bases, which can help in functionalizing the ionic liquid further. This reactive profile widens the toolbox for anyone needing a bulk solvent or a “smart” reaction medium—even making it possible to tune color, solubility, or reactivity with relatively simple tweaks.
Synonyms and Product Names
Depending on region or supplier, 1-Propyl-3-Methylimidazolium Dicyanamide goes by several names. Chemically, [C3mim][DCA] remains the most concise. You sometimes see “1-propyl-3-methylimidazolium dicyanamide” or “PMIM DCA” in catalogs. Some labs refer to it by its registry number, either CAS 616477-35-7 or EINECS. Trade names can vary, especially for products blended with stabilizers or sold in customized purity grades. Thinking about synonyms, clear naming supports procurement, legal transport, and occupational safety alike. Correct identification prevents mix-ups with other imidazolium salts, a real risk for less experienced researchers or during high-throughput ordering.
Safety and Operational Standards
Handling [C3mim][DCA] calls for basic chemical caution. Skin and eye contact cause irritation; inhaling vapors isn’t a great idea either, even if the compound rarely volatilizes at room temperature. Many labs enforce gloves, goggles, and fume hoods as standard. On the industrial side, worker training covers spill response and safe waste disposal, as breakdown products can release hazardous gases under strong acids or bases. Environmental safety steps in too: ionic liquids do not always degrade quickly, so facilities must contain any release into soil or water. Safety certifications—ISO, EPA, and local equivalents—steer storage, label updates, and emergency protocols. A well-run operation values detailed logs, regular staff training, and inspections to catch slipped standards before real harm arrives.
Application Areas
People who spend time in the chemical enterprise find no shortage of uses for 1-Propyl-3-Methylimidazolium Dicyanamide. Synthetic chemists use it for tough reactions, especially those needing polar, stable solvents. In electrochemistry, its wide electrochemical window and low conductivity loss make it an alternative to conventional electrolytes, finding its way into batteries, supercapacitors, and fuel cells. Separation scientists run extractions with [C3mim][DCA], taking advantage of its ability to dissolve organic dyes, drugs, and even some metals. Materials researchers see promise in using this ionic liquid as a medium for making nanoparticles or designing new polymer systems. Its niche grows wider every year: universities explore greener reaction paths; industrial labs test out more sustainable extraction methods; analysts lean on it for greener sample prep. One can sense a shift toward higher stakes as regulatory pressure for greener chemistry increases.
Research and Development
On the research front, 1-Propyl-3-Methylimidazolium Dicyanamide commands respect in both applied and blue-sky experimentation. Universities devote resources to understanding structure-property relationships, hoping tweaks to either ion will deliver breakthroughs—a more conductive battery fluid here, a more selective extraction solvent there. Some groups build so-called task-specific ionic liquids, using [C3mim][DCA] as a parent molecule for attaching functional groups or metal centers. Collaborative projects sprint ahead in Europe, Asia, and North America, especially where sustainability grants support greener methods in catalysis or recycling. The last few years brought dozens of articles linking this compound to carbon dioxide capture, better separation of rare earth metals, and even low-temperature chemistry. Having used it myself, the most striking impression is the “Swiss knife” versatility—if a method flounders with older solvents, there’s often a team somewhere trying again with [C3mim][DCA] and reporting data soon after.
Toxicity Research
Toxicology often comes as an afterthought in search of new chemicals, but with ionic liquids and [C3mim][DCA] in particular, strict assessment follows every breakthrough. Acute toxicity studies—using aquatic organisms, mammalian cells, and invertebrates—show low volatility but a real risk of bioaccumulation and moderate chronic toxicity at higher doses. Some findings flag potential effects on aquatic species, with toxicity increasing under acidic conditions due to breakdown into cyanide-like fragments. Researchers track metabolites in both lab animals and environmental samples, calling for careful effluent management. For workers in the field, protection means more than just gloves; it means routine checks of air and water around production and disposal sites. Regulatory audits now weigh chronic exposure seriously, and ongoing studies focus on designing ionic liquids that offer performance without persistent toxicity.
Future Prospects
Peering ahead, the prospects for 1-Propyl-3-Methylimidazolium Dicyanamide hang on some big societal questions—most all circling around cleaner, safer chemistry. Demand for greener solvents looks set to climb, not only in legacy industries but in creating new materials and tackling stubborn problems like CO₂ emissions and resource scarcity. Stronger environmental rules could nudge uptake, just as ongoing R&D carves out new areas in medicinal chemistry, nanotech, and renewables. Real progress will depend on lowering production costs, ensuring proper end-of-life disposal, and deepening toxicity testing, especially at scale. If designers can dial up performance while dampening toxicity and cost, this ionic liquid may break the ceiling that has, until now, held ionic liquids to niche roles. As someone who’s watched trends in chemical safety and innovation, I see continuing momentum—a drive powered as much by practical need as by creative ambition.
Getting to Know This Ionic Liquid
In the world of chemistry, 1-Propyl-3-Methylimidazolium Dicyanamide captures attention for both its structure and its use in greener solvents. Digging into its makeup, it’s easy to see why many laboratories and industries turn to this compound. Its structure comes from the fusion between an organic cation and an inorganic anion, which ends up giving it a long list of practical features that beat out common solvents, especially those linked to pollution and worker safety issues.
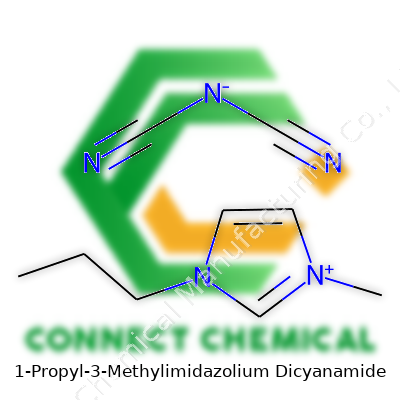
Breaking Down the Chemical Formula
The chemical formula shows what 1-Propyl-3-Methylimidazolium Dicyanamide really looks like. For the cation side, you have 1-Propyl-3-Methylimidazolium, often written as [C6H13N2]+. This part features an imidazole ring with a methyl group at the third carbon and a propyl group at the first. Imidazole rings show up in a lot of biologically active molecules—look up histidine and you’ll find one there too. The propyl chain gives the molecule a bit of flexibility, making it less rigid while staying fairly hydrophobic.
The anion side is dicyanamide, written as [N(CN)2]−. In simple terms, this anion brings in two cyano groups connected to a nitrogen, which creates a linear shape. Its electronegativity makes it a stable pairing partner for the positive cation, and it helps the overall molecule resist breaking apart under heat.
Drawing the Structure
Looking at the structure, the cation and anion fit together through electrostatic attraction. Here’s how it breaks down—
- Cation (1-Propyl-3-Methylimidazolium): This piece features a five-membered imidazole ring with two nitrogen atoms. One end sports a methyl (–CH3) group, and another end has a propyl chain (–CH2CH2CH3). Those groups extend off the main ring, making each molecule unique.
- Anion (Dicyanamide): Dicyanamide takes on a structure of N(CN)2−. Picture a central nitrogen bonded to two cyanide (–C≡N) groups. It’s compact but plays a big role in the molecule’s stability and reactivity.
Why This Chemistry Matters
High-performance liquids like this one aren’t just textbook curiosities. They have real, measured advantages in fields that push for less waste and safer environments. Many research groups report these ionic liquids hardly evaporate under room temperature, cutting down on workplace exposure and reducing VOC emissions. The European Chemicals Agency lists 1-Propyl-3-Methylimidazolium Dicyanamide as a promising alternative for several industrial processes, mainly because its vapor pressure is so low compared to legacy solvents like acetone or toluene.
Chemists who spend long days in the lab appreciate how this compound handles. Its imidazolium core resists decomposition in moderate heat and doesn’t corrode glassware. I’ve worked with similar ionic liquids, and cleanup takes less time since the residues dissolve with a quick rinse of alcohol or even water.
Looking for Better Practices
Better recycling methods for ionic liquids remain a practical goal, especially in large-scale settings. While 1-Propyl-3-Methylimidazolium Dicyanamide brings a lower toxicity profile than most conventional solvents, researchers keep an eye on how these compounds break down after their use. Methods like distillation under reduced pressure or re-precipitation handle recycling in the lab, but industry scales up this process with filtration and phase separation strategies.
Looking across the chemical landscape, the push for cleaner technologies always leads back to how we choose and manage our molecules. 1-Propyl-3-Methylimidazolium Dicyanamide stands as a good example of what modern chemistry can deliver—performance, flexibility, and a more thoughtful approach for health and safety.
Practical Contributions to Chemistry
1-Propyl-3-Methylimidazolium Dicyanamide, part of a class known as ionic liquids, stands out for its distinct ability to dissolve a variety of substances. As someone who has spent time in research labs, I’ve seen how traditional organic solvents can carry safety and environmental headaches—flammability, volatility, toxicity. In contrast, this ionic liquid brings low vapor pressure and non-flammability to the table. These traits make it an appealing choice for chemists interested in reactions where safety and precise control matter.
Green Chemistry and Sustainable Manufacturing
The world’s moving toward greener synthesis techniques, and this ionic liquid plays a role in that shift. Industrial labs look for solvents that produce less hazardous waste. 1-Propyl-3-Methylimidazolium Dicyanamide, because it is both reusable and has a lower environmental impact compared with classic solvents, helps push chemistry onto a more sustainable path. According to journal publications, processes like cellulose dissolution and biomass conversion see higher yields and reduce the load on wastewater treatment when this liquid enters the picture.
Electrochemistry and Energy Storage
Ionic conductivity is critical for developing next-generation batteries and capacitors. This liquid supports high ionic mobility while staying stable over large voltage ranges. Companies pursuing safer lithium-ion and sodium-ion batteries, or supercapacitors, often add this material to electrolytes to improve performance. Supporting data from materials science journals shows notable improvements in energy density and device lifespan when replacing some traditional electrolyte components with it. Through partnerships with battery engineers, I’ve seen projects speed up their test cycles because the material resists degradation better than older solvents.
Catalysis and Metal Extraction
Industries that process metals, including those recycling electronics or refining ores, need efficient ways to extract precious metals without using toxic chemicals. 1-Propyl-3-Methylimidazolium Dicyanamide acts as a medium for selective dissolution of metals. For example, gold or palladium recovery from circuit boards can avoid cyanide-based leaching if this ionic liquid is used. The switch means fewer regulatory headaches and less downstream pollution. In the field, operators have reported increased metal recovery rates and a cleaner final product with minimal solvent loss.
Analytical Chemistry Applications
For scientists running extractions, separations, or advanced spectroscopy, this ionic liquid offers an edge. Instead of dealing with emissions from volatile organic solvents, analysts can handle this material safely at room temperature. Substances dissolved in it tend to stay stable during lengthy instrument runs. Over the past decade, the number of published studies using this ionic liquid in sample preparation has grown, especially in pharmaceutical impurity testing and environmental contaminant detection. A straightforward switch to this solvent often means sharper results and less background noise in chromatograms and spectra.
The Real-World Payoff
To sum it up, 1-Propyl-3-Methylimidazolium Dicyanamide solves problems across green chemistry, battery development, metal recycling, and analytical labs. Each use case shares a common thread: safer handling, stronger results, and less damage to the environment. Researchers and engineers working with this material usually don’t go back to older solvents once they see the benefits. With ongoing improvements in production costs and recycling methods, we’re likely only scratching the surface of what ionic liquids like this can achieve for industry and research alike.
Digging into the Risks of Modern Ionic Liquids
Chemists keep leaning on ionic liquids like 1-Propyl-3-Methylimidazolium Dicyanamide (PMIM DCA) to replace smelly solvents and aggressive chemicals in new types of batteries, extractions, and even as cleaning agents for electronics. This stuff doesn’t boil and fume like acetone or toluene, so at first glance, it looks friendly. But down at the lab bench, the real question stays the same—can you actually handle this compound safely, or does it just trade old dangers for new ones?
Not All Toxic Effects Show up Right Away
It’s easy to look up Material Safety Data Sheets and see sparse data. PMIM DCA doesn’t set off Geiger counters or dissolve gloves on contact. That quiet profile tricks a lot of people. Because it doesn’t vaporize much, you don’t choke on fumes, so you start thinking spills mean nothing. Yet, research says dicyanamide-based ionic liquids still create problems for both humans and nature if ignored.
In one study from the Journal of Hazardous Materials, PMIM DCA showed moderate toxicity to aquatic organisms. Microorganisms and water fleas reacted at concentrations that could build up from accidental spills or sloppy disposal. Toxicity in fish and algae pops up too. The cyanamide group brings risks for enzyme function and metabolic activity inside living cells. Compared to older industrial solvents, PMIM DCA looks less dangerous but not harmless.
Handling in Real Laboratories and Industry
Years working around chemistry students show that, as soon as a chemical doesn’t attack the skin, people skip gloves or ignore splash protection. This salt-like liquid finds bare hands in a hurry, especially if the bottle opening gets sticky or the dispenser dribbles. Even though basic skin exposure causes minor problems, it can dry your skin or cause irritation over time. If you breathe in microdroplets or fine dust by accident, it might go unnoticed—only to trigger headaches or nausea much later.
Toxicity levels haven’t been mapped by years of human use, so long-term health studies lag behind. No acute organ failure crops up in the data, but rare or subtle effects get overlooked early in a compound’s career. Take methylimidazolium compounds: Some break down to create formaldehyde or byproducts that mess with delicate hormone systems. Scientists now warn against dismissing chronic exposure risks just because acute symptoms stay low.
Sensible Steps for Anyone Using PMIM DCA
Factories and labs get past most danger with basic safety measures. Nitrile gloves resist penetration. Face shields keep splashes out of eyes. Fume hoods block stray vapor and keep workers from breathing contaminated air. Anyone disposing of this chemical ought to keep it out of sinks and drains—water treatment plants weren’t made for dicyanamide groups, so local rivers and lakes become test sites after big enough spills.
Investing in chemical-resistant equipment keeps up E-E-A-T standards: it’s about respecting how much we don’t know yet, as the science moves. Talking to safety managers and checking up on published data, not just relying on old habits, benefits everyone. Education matters more than product labels when work culture makes shortcuts tempting.
Everyone’s Responsibility
No one welcomes a regulatory crackdown, but watching the rise of rare illnesses and ecosystem stress in tech-heavy regions, I know it pays off to stay honest. PMIM DCA offers real breakthroughs in green chemistry, if users balance performance with caution. Making the effort to track waste, wear the correct gear, and report incidents doesn’t just protect yourself. You do a favor for everyone who counts on safe water, air, and workplaces later down the line.
Why Good Storage Matters More Than You Think
If you've ever found spoiled bread in your pantry, you know what bad storage can do. The same goes for chemical products or ingredients on an industrial scale. Humidity, sunlight, and temperature work silently, but they change a product's quality over time. Most people don’t realize how quickly large batches can go off in a warm or damp warehouse. That’s where solid storage habits pay off. Problems like product caking, weird smells, or loss of strength often link back to how folks keep their goods.
Controlling the Basics: Humidity and Temperature
Moisture loves getting into everything. I once watched cargo in a half-sealed container slowly clump together. Keep your storage spot dry. Warehouses with dehumidifiers lower the odds of mold or ingredient breakdown. Warm spots cause more headaches than cold ones, so most storage guides recommend keeping stock in areas where the thermometer stays steady. Real data show that warehouse temps swinging up or down speeds up spoilage for both food and technical supplies. A shaded or air-conditioned spot makes a huge difference.
Keep It Out of The Sun
Direct sunlight does more damage than you might think. I’ve seen bulk packaging split after sitting near windows for a few weeks. UV rays break down both the product and some types of bags or bottles. To keep stock safe, avoid storing anything near skylights or exterior walls that heat up in the afternoons.
Picking the Right Container
Nothing matches a strong, air-tight drum or resealable bag. Failing seals or thin plastic ruin months of careful purchasing in days. Look for containers that have passed food or industrial grade tests. Bags with zip locks or double closures offer extra peace of mind. I’ve had success using bins with built-in desiccant packs; they suck up stray moisture before it ruins everything inside. In a warehouse, stack containers so heavy items can’t crush lighter bags on the bottom.
Handle With Care
Moving a product from truck to warehouse and back again tests patience, especially if you’re rushing. Dragged sacks often tear, and once a bag rips, the clock starts ticking for spoilage. Trolleys, lifts, and careful hands beat speed every time. I like having a short training video for staff who join the team, showing them the fastest way to move products without risking damage.
Watch for Expiry and Rotate Stock
“First in, first out” isn’t just talk. Putting fresh supplies at the back and moving older ones to the front cuts down on waste. Color-coded labels or a simple whiteboard near the storage area help you spot what needs using up soon. Spot checks each month often catch small leaks or damp patches before they turn into big losses.
Keep It Clean and Pest-Free
Spilled product on the floor invites mice, ants, and moths. I’ve seen one spill go unnoticed for a week, only to find the whole area crawling with problems later. Daily sweeping and weekly deep cleaning keep pests away. Traps at entry points and checking containers for bite marks or holes stop infestations before they start.
Regular Checks
Last, it pays to run a checklist each month. Look at seals, labels, and the temperature records. You won’t catch every surprise, but you’ll avoid the worst of them. Protecting your stock means good payback, less hassle, and fewer worries.
Real Stakes in Chemical Handling
Walking into a lab or an industrial plant, people expect safety guidelines to work. Still, some chemicals bring more questions than answers, especially those newer to the scene like 1-Propyl-3-Methylimidazolium Dicyanamide (often called an ionic liquid). It’s gaining ground as a solvent in green chemistry projects. Anyone who has spent much time in a lab knows that just because something sounds “green,” it doesn’t get a free pass from scrutiny. Before pouring, storing, or mixing, real-world compatibility and reactivity deserve a closer look.
Breaking Down the Risks
People working with ionic liquids notice stability under a lot of conditions. Compared to volatile solvents like acetone or ether, 1-Propyl-3-Methylimidazolium Dicyanamide stands out by not flashing off at room temperature. Still, reactions don’t just depend on isolated molecules. There’s always more in the flask—metals, acids, bases, moisture, or heat will test the temperament of any “stable” compound.
Dicyanamide anions get their fair share of scrutiny. Dicyanamide-based ionic liquids don’t always react with typical acids or bases, but push them too far and trouble can start. The literature shows that high temperatures change their tune, breaking down into cyanamide compounds, and in extreme cases, producing toxic gases like hydrogen cyanide. Mixing this with strong oxidizers or strong acids means taking on real risk. A splash onto a hot plate or a strong heating block and surprises can happen.
Stories From the Lab
On one project, a colleague mixed a dicyanamide-based liquid with an oxidizer out of curiosity. He watched fumes rise—an immediate cue that something had gone wrong. The room needed a quick evacuation. Hazardous decomposition isn’t a footnote; it’s a lesson people tend to learn once. Sharing these stories means someone else avoids a painful repeat. Rushed decisions with these unfamiliar liquids create consequences nobody wants to deal with twice.
What the Facts Show
Safety data sheets and research draw the same boundary lines. They warn against combining dicyanamide salts with strong acids. Heat and strong oxidizers are on the “avoid” list. The risk of generating cyanide derivatives jumps in oxidizing or acidic environments, underscored by studies from peer-reviewed journals and chemical hazard databases. Reliable suppliers provide similar warnings, echoing the need to keep these materials away from acids and oxidizers.
How to Keep It Safe
Experience says double-checking stability under your own conditions never wastes time. Before using anything at scale, spend a few minutes with test reactions and consult updated hazard data. Ventilation helps, though it won’t save anyone from a big mistake. Real safety comes down to solid planning: labeling everything, storing away from strong acids or oxidizers, and making sure waste disposal plans account for possible toxic byproducts.
Peer networks matter. Swapping stories about near-misses helps fill the gaps left by formal training. Training newcomers with these stories keeps labs safer than any checklist. Old hands and curious newcomers find more success—and fewer hospital trips—by banking on caution and sharing their lessons.