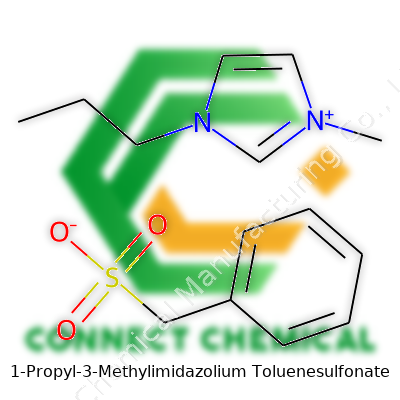
1-Propyl-3-Methylimidazolium Toluenesulfonate: A Comprehensive Commentary
Historical Development
The roots of ionic liquids like 1-Propyl-3-Methylimidazolium Toluenesulfonate reach back to the early investigations in the mid-20th century, when researchers tried to understand salt compounds that stayed liquid at room temperature. Early progress stalled for decades, mainly because synthetic methods sometimes produced unstable products. In the 1990s, driven by the desire for green chemistry solutions and alternatives to volatile organic solvents, research into ionic liquids picked up steam. The pairing of the imidazolium cation with toluenesulfonate anion opened new possibilities for tailored solvent systems. It took persistent organic chemists willing to sift through the messiness of trial and error to identify practical routes for producing stable, usable salts fit for laboratory and industrial work. Today, the world of ionic liquids looks remarkably different, with these substances showing up across countless research settings. New applications trace back to those early experiments in small, often overheated university labs.
Product Overview
1-Propyl-3-Methylimidazolium Toluenesulfonate stands out within the family of ionic liquids for its low volatility and its ability to dissolve a wide array of organic and inorganic materials. Built around the imidazolium ring, its structure delivers stability and versatility. The compound’s character draws not just from the core imidazolium but also from the tuning of its alkyl chains and pairing with the bulky toluenesulfonate. Chemists working with advanced separations, catalysis, and battery systems rely on the unique solvating abilities and ionic conductivity of this liquid. It is less about showy claims and more about dependable performance under real-world laboratory conditions. Both academic research and scale-up trials reflect appreciation for its adaptable solvation profile, especially in challenging synthetic transformations and material processing applications.
Physical & Chemical Properties
The liquid stays fluid at room temperature, a feature that has practical value in labs lacking specialized heating or cooling gear. Its colorless to pale yellow appearance lets users quickly spot contamination. With a melting point well below 25°C and a density close to 1.1 g/cm³, handling rarely feels cumbersome compared to denser salts. The compound resists volatilization, so spill control is less stressful than with solvents that are quick to evaporate. Conductivity falls within the typical range for similar ionic liquids, which translates into reliable charge transport in electrochemical experiments. Its thermal stability often exceeds 200°C, making it suitable for processes where organic solvents would simply break down. Solubility in water and many polar organics further increases its appeal for process development, especially in areas like extraction and synthesis where versatility counts.
Technical Specifications & Labeling
Manufacturers provide 1-Propyl-3-Methylimidazolium Toluenesulfonate with specifications targeting high purity—typically over 99%. Trace metal and halide content often fall below strict thresholds, reflecting the demands of catalyst and electronics markets. Labels include clear batch numbers, production date, expiration timelines, and storage guidelines, usually recommending a cool, dry, and light-protected environment. Hazard statements address skin and eye irritation, so the usual personal protective equipment—gloves, goggles, and lab coats—remains the best way to avoid regret. Customers expect suitable documentation, including Certificates of Analysis and Safety Data Sheets with instructions on spill response and waste disposal. I have found greater trust among research partners when suppliers demonstrate traceability and consistent quality, a detail often overlooked until problems emerge.
Preparation Method
Synthesizing 1-Propyl-3-Methylimidazolium Toluenesulfonate starts by alkylating methylimidazole with propyl halide under controlled, inert conditions to give the target cation. Purification—removing excess reagents and minimizing residual halides—takes up the bulk of the post-reaction work. The salt metathesis step, where the cation meets sodium toluenesulfonate in aqueous or organic media, drives formation of the final product. Several extraction and washing phases help remove by-products. Drying under reduced pressure ensures removal of water, which otherwise reduces performance in many uses. Over my years working in organic synthesis, hands-on practitioners know that standardization matters, as even small amounts of impurities can impact catalytic or electrochemical outcomes. Lab notebooks packed with observations from small-batch runs have led to better yields and higher purity, supporting both research reproducibility and commercial scale-up.
Chemical Reactions & Modifications
This ionic liquid acts as both a solvent and a participant in organic transformations. Its stable imidazolium cation resists common nucleophiles and bases, so it often doesn’t interfere with standard reaction pathways. Modifications typically occur on the side chains or the anion. Swapping the toluenesulfonate for other aromatic sulfonates can be performed using salt exchange processes. Chemists have tethered transition metal catalysts to the imidazolium ring, producing catalyst systems that stay in the ionic phase and simplify recovery. I’ve seen such tweaks drive up efficiency, cut waste, and make recycling more practical in pilot plant trials. The liquid’s resistance to reduction and oxidation also gives it a place in advanced battery research, where its long shelf life and electrochemical window enhance safety compared to older systems built with organic solvents.
Synonyms & Product Names
1-Propyl-3-Methylimidazolium Toluenesulfonate also shows up under trade and laboratory names like [PMIM][Ts], [C3MIM][OTs], and 1-propyl-3-methylimidazolium p-toluenesulfonate. In research catalogues, users may see CAS numbers such as 288085-32-5, or product codes assigned by suppliers focused on specialty chemicals. Through years of ordering from different suppliers, confusion about names often leads to hiccups in procurement. Double-checking structure and documentation always pays off when switching between vendors or working across international borders.
Safety & Operational Standards
Safety assessments highlight the need for deliberate handling. Direct skin and eye contact triggers irritation, so sensible use of gloves, eye shields, long sleeves, and fume hoods reduces risk. In case of spills, I always reach for absorbent materials and avoid water if large volumes are involved, instead isolating and disposing of waste by institutional guidelines. Storage in tightly sealed containers kept away from sunlight and extremes of heat protects stability and lowers accidental exposure. Institutional safety committees expect clear signage and updated safety data, and users trained in chemical hygiene practices. Emergency procedures should reflect the compound’s physical properties, so prompt reporting and cleanup prove more effective than procedural shortcuts. Long lab hours remind me that a few minutes of diligence with protective gear pay back in health and productivity.
Application Area
Industries and research groups pull 1-Propyl-3-Methylimidazolium Toluenesulfonate into roles such as catalyst immobilization, advanced separations, and battery electrolyte development. Organic chemists value its solvating power in cross-coupling and sustainable oxidation reactions, often opting for it in order to run higher-yield processes without the hazards tied to volatile solvents. In materials science, it boosts dissolution of polymers like cellulose, supporting progress in bioplastics and more efficient biomass conversion. Electrochemical setups benefit from its high ionic conductivity and electrochemical stability. Over many years working with process engineers, I have found the liquid’s unique mix of properties unlocks projects aiming for lower emissions, safer working conditions, and greater efficiency.
Research & Development
Research on 1-Propyl-3-Methylimidazolium Toluenesulfonate covers both fundamental science and commercial application. Innovators keep testing new side chains, hybrid systems with nanoparticles, and blends with other ionic liquids to tackle practical questions—can the material endure tougher operating environments, or enable extraction of rare earths from industrial waste at scale? Research labs that share findings with industry partners spur more rapid progress. I have noticed that partnerships lead to new applications faster than solo academic efforts. As industry ramps up interest, standards for purity, stability, and life-cycle analysis become more rigorous, shifting development toward robust, scalable processes.
Toxicity Research
Toxicology studies for 1-Propyl-3-Methylimidazolium Toluenesulfonate still leave key questions unanswered. Early work shows moderate aquatic toxicity and some risk to soil microorganisms, a reminder that claims about “green” solvents demand careful evidence. Acute toxicity through skin or inhalation uptake remains below the level of most volatile organic solvents, but chronic effects remain under investigation. Efforts to trace breakdown products in environmental systems lead to mixed results: some studies note bioaccumulation, others describe rapid degradation under the right microbial or chemical conditions. Laboratory safety practices, designed with incomplete data, default to caution. Over the years, reviewing risk assessments and regulatory filings confirms the need for active monitoring and development of safer disposal strategies.
Future Prospects
Interest in 1-Propyl-3-Methylimidazolium Toluenesulfonate continues to grow, driven by the need for more sustainable chemistry, safer laboratory practices, and higher-efficiency industrial production. As more teams push for circular economy solutions, the liquid’s recycling potential and performance in closed-loop systems takes center stage. New synthesis routes, using renewable feedstocks and less energy-intensive conditions, could further cut the environmental and economic cost of adoption. Cross-disciplinary projects often spark the best ideas—combining insights from analytical chemistry, toxicology, and process engineering usually yields real progress, not just in lab-scale breakthroughs but in scalable, safer commercial solutions. As regulatory agencies and industry watch environmental and safety impacts, work continues on designing less toxic variants and on systems capable of recovery and reuse on a global scale.
A Closer Look at This Ionic Liquid
Chemists and engineers talk often about ionic liquids, especially ones like 1-Propyl-3-Methylimidazolium Toluenesulfonate. This long-named salt has a knack for solving problems that stump more common chemicals. Liquid at room temperature, it acts less like water and more like a magic trick: able to dissolve stubborn compounds, sit happy with metals, or carry out unusual reactions. People working in synthesis look to liquids like this for their stability. They don’t catch fire easily and tend to avoid evaporating into the air. That means safer benchtops and less drama with regulators trying to keep the workplace healthy.
Behind the Scenes in Green Chemistry
The push for greener chemistry isn’t just about ditching oil. It’s a hunt for techniques that give the same results without nasty byproducts. Traditional solvents generate canisters of hazardous waste every week, especially on an industrial scale. Using 1-Propyl-3-Methylimidazolium Toluenesulfonate, researchers make progress toward more sustainable processes. If you talk with a lab that tried swapping out regular solvents for this, stories of easier recycling and fewer emissions crop up again and again. The ionic liquid’s low volatility means less inhalation risk for the crew handling the setups. Reports show a marked drop in exposure to fumes, which makes real difference for everyone’s long-term health.
Catalysis and Electrochemistry
Colleagues in catalysis rave about the way these liquids let them run reactions faster and sometimes even skip extra purification steps. Instead of reaching for toluene or ethers, they turn to this ionic medium. Take Suzuki coupling reactions—switching in this liquid has boosted yields and saved time in my group, making a once-messy job a single-pot operation. People using electrocatalytic setups also like how these salts conduct electricity. They support steady currents for plating metals or splitting water, all without rusting the electrodes into useless chunks. Numbers from recent studies show an uptick in energy efficiency, and costs tend to drop once you stop replacing corroded gear.
Working with Biomaterials and Extraction
Extracting value from plants or waste streams gives headaches unless you pick the right solvent. Since ionic liquids can dissolve both polar and nonpolar stuff, workers in bio-refineries use them for lignin extraction, cellulose dissolution, and recovering important chemicals with less fuss. Teams I’ve visited have explained how this liquid lets them squeeze more product from less starting material. It’s not a one-size-fits-all tool, but its range beats most conventional solvents. This plays out in food engineering, too, where flavor compounds and pigments get pulled out without the risk of petrol residue tagging along.
Where Safety and Scale Meet
The question becomes less about whether the chemistry works and more about if it scales. Regulatory filings and fire codes edge out the classics for ionic liquids in some countries. People report fewer issues with hazardous goods shipping, since these liquids escape the most severe hazard classifications. Tighter controls on solvent emissions and workplace exposure rules mean companies want reliable, reusable, and less volatile materials. Data from pilot plants show that switching to ionic fluids slashes cleanup times and reduces the bill for personal protection.
Lab work finds its best footing in solutions that honor both creativity and real-world limits. 1-Propyl-3-Methylimidazolium Toluenesulfonate stands out as a workhorse where safety, efficiency, and environmental benefit overlap. In my time at the bench, nothing beats finding a tool that pulls its weight in more than one part of the process. This liquid lands squarely in that sweet spot.
The Building Blocks: Understanding the Names
Chemistry class memories come rushing back with a name like 1-propyl-3-methylimidazolium toluenesulfonate. It sounds intimidating until you break it down. Imidazolium sets the stage—a five-membered ring with two nitrogens. Slap a propyl group on one nitrogen, a methyl group on the other, and you’ve got 1-propyl-3-methylimidazolium. On its own, that's a cation, or positively charged ion. Then there's the partner: toluenesulfonate, an anion that brings the sulfonate group onto a toluene ring.
The Importance of the Actual Formula
The chemical formula isn’t tough to track down, but understanding its importance takes a bit more effort. The cation, 1-propyl-3-methylimidazolium, is C7H13N2+. The anion, toluenesulfonate, is C7H7SO3-. Put them together and you get C7H13N2+ C7H7SO3-, often written as C14H20N2O3S. Seeing the formula, you realize something: this isn’t only about learning to draw carbon rings and number atoms. It’s about knowing the basic structure influences everything from how it dissolves in water to how it interacts with other substances in the lab.
Why Formulas Like These Shape Progress
Ionic liquids like this one have a reputation for shaking up the chemical world. They pop up in green chemistry, battery tech, and organic synthesis. The unique properties of an imidazolium cation paired with toluenesulfonate help researchers cut down on volatile organic solvents, which can trash the environment and pose real safety risks. I saw this at work in an academic lab, switching from traditional solvents to these salt-like liquids for extractions and separations. The entire vibe in the lab changed—fewer fumes, less waste.
The chemical formula becomes more than a relic from textbooks; it is a passport to a world of safer processes and lower environmental impact. The room-temperature liquid nature of this compound boils down to the quirky balance of its structure. Cations and anions, once dismissed as simple building blocks, determine whether you walk away with a toxic mess or a manageable solution.
The Need for Responsible Handling and Future Solutions
Chemicals with long names and complex formulas get misused or overlooked when people skip the legwork of understanding what's actually in the bottle. Transparent labeling, access to full material safety data sheets, and proper training keep everyone safer. The formula opens doors to innovation, but it also accepts no shortcuts—using chemicals responsibly keeps both researchers and the environment from harm.
Real potential sits in using these chemical structures for cleaner processes and smarter materials. Scrutinizing the formula gives researchers a chance to tweak one group or swap an ion for another, creating whole new families of useful substances. Instead of blind faith in the latest trend, a little chemistry know-how goes a long way. The formula, C14H20N2O3S, stands as both a blueprint and an invitation to think a few steps ahead, driving research to be not only more effective but safer for those of us who spend our days—and sometimes our nights—in the lab.
Understanding the Chemical Itself
Many folks working in chemistry or material science labs see longer chemical names like 1-Propyl-3-Methylimidazolium Toluenesulfonate and their guard goes up. It’s not found on your local grocery shelf; this is a specialty ionic liquid often made and used in professional labs, typically to carry out reactions in ways water or oil can’t. The imidazolium family is well-known in the green chemistry world because some of its compounds let you run chemical processes more efficiently. Still, the question comes down to real-world risk: is this compound hazardous or toxic if you actually have to handle it?
What Research Says About Hazards
A lot of ionic liquids, including those based on imidazolium cations like this one, show remarkably low volatility. You won’t get a blast of fumes walking past an open bottle, which is a plus in any lab. People often think of these liquids as “safer solvents” for that reason, especially compared to classic choices like benzene. Still, low volatility doesn’t mean non-toxic. Small changes in chemical structure shift toxicity by a mile—sometimes in ways that only pop up after long-term or repeated exposure.
Studies have shown that the imidazolium bit can irritate skin and eyes if you spill some during transfer, and accidental splashes happen even to careful folks. No matter what, good lab practice means gloves and eye protection, and I’ve seen too many people trust a chemical just because it’s called an “ionic liquid.”
Digging through available safety data, there’s little evidence of acute toxicity from a drop or two on the skin, but that doesn’t translate to “safe.” The toluenesulfonate part comes from toluene, which has a reputation for causing headaches and dizziness after inhalation in its pure form. Yet, combining it with the imidazolium cation shifts its behavior—making it far less volatile and less able to cause the same short-term symptoms as toluene itself. Long-term exposure data remains thin. Without robust, repeated studies on bioaccumulation and breakdown in the human body, caution wins out for most professionals.
Environmental and Occupational Concerns
Even “green” solvents have hidden impacts. Research out of Europe looked at how some ionic liquids can linger in water after disposal because wastewater treatments fail to break them down fully. That might not hit home right away, but these persistent compounds could damage aquatic life, building up in riverbeds or making their way up the food chain. Some studies point to imidazolium-based liquids stressing small crustaceans and algae. Decisions made on a bench in one lab ripple out farther than most people realize.
Practical Steps for Handling Risks
Safety always starts with reliable handling. Keep all work involving unfamiliar chemicals in well-ventilated fume hoods, limit exposure, and store compounds in containers built for chemicals. Lab training easily gets skipped when deadlines push, but knowledge about specific substances matters. If a spill happens, know what to reach for—absorbent pads, not bare hands.
For broader solutions, labs and manufacturers need to lobby for more public toxicity data on new and existing ionic liquids. More transparency leads to safer substitution choices and proper disposal guidelines. Pushing for rigorous hazard testing before scaling up production isn’t just bureaucracy. It matters for those making, using, and cleaning up chemicals—sometimes long after everyone else has gone home.
Why Proper Storage Matters in Every Lab
Someone working in a lab can spot trouble pretty quickly when basic safety gets ignored. Think about 1-Propyl-3-Methylimidazolium Toluenesulfonate—a mouthful in name, but in practice, just another job for careful handling. Ionic liquids, including this one, change the game with their unique properties, but they also bring real risks if you shove them on a shelf and walk away.
Keeping Stability on Your Side
Ionic liquids rarely evaporate at room temperature, which seems handy at first glance. Over time, sticking the bottle next to a window or letting it sit through temperature swings messes with purity and performance. Sunlight doesn’t do chemicals any favors. That “oh, it’s just warm in here” feeling can speed up breakdown or even start reactions you didn’t sign up for. Direct light breaks some ionic liquids down, which pulls you away from clean, reliable results. Most reactions in the bench world ask for predictable inputs.
Get the Humidity Under Control
Humidity sounds harmless unless you’re storing chemicals that thrive on dryness. Water can sneak in and change how this salt behaves. In my own experience, seeing products ruined by picking up moisture in a supposedly “clean” room hits differently when you’re the one left cleaning the sticky mess or explaining flagged sample results to a supervisor. Desiccators and tightly sealed bottles aren’t overkill; they’re the regular price of keeping things in working order. Moist environments don’t just risk ruined samples; they can contribute to mold growth and strange smells.
You Don’t Want Contamination—Not from the Air, Not from Your Hands
Open containers attract dust and all the bits in the air nobody ever thinks about until it’s too late. Pouring out just a little and capping the rest right away gets overlooked—all it takes is a single shortcut to lose a batch that took a week to arrive.
Don’t Ignore Compatibility with Its Container
Plastic looks cheap and easy, but not all containers stand up to ionic liquids. Glass usually holds up under repeated use and doesn’t react. I remember a colleague using an old plastic bottle to “save time”—we spent double that time dealing with cloudiness and ruined stock. Most ionic liquids, including this one, play much safer with glass. Label the container well with the storage date and the supplier details, not just because it looks official, but so nobody guesses six months later what’s inside.
Fire & Safety Risks Deserve Respect
Some ionic liquids show surprisingly flammable behavior if handled carelessly. Keep them away from open flames and strong oxidizers. Store in a spot lined with chemical-resistant shelving. Spills can trigger reactions, so having spill kits and goggles nearby isn’t about compliance—it’s about coming home with your vision and health intact.
Practical Steps Everyone in the Lab Should Know
- Keep the compound tightly sealed in chemically resistant glassware.
- Label everything clearly with batch dates and hazards.
- Store in a cool, dry, and dark place. No sun, and keep temperatures steady, aiming below 30°C.
- Use desiccants to fight off moisture any time the room isn’t fully climate-controlled.
- Place containers away from acids, bases, and anything with a strong oxidizing punch.
- Wear gloves, goggles, and a coat every time you use it. Don’t slack on ventilation.
Building Habits That Protect People and Results
Some basics never change. If you keep shortcuts out of chemical storage, the day stays safer and the science stays solid. It’s not about paranoia; it’s about respecting the power of what’s on the shelf—so the next team, or the next shift, finds everything exactly as it should be.
Stepping Up Green Chemistry
1-Propyl-3-Methylimidazolium Toluenesulfonate lands on the workbench when industry wants to move past harsh solvents. Many chemical processes have relied on volatile organic compounds for years, but their fumes and residue spell trouble for both workers and the environment. This ionic liquid stands out, offering a safer way to do the same job—no more headaches from strong smells, and less impact on water and air once everything’s cleaned up. In my own lab experience, swapping out these older chemicals for the ionic kind really opened doors to safer, more reliable work, especially for folks handling reactions every day.
Pushing Forward in Synthesis
Synthetic labs turn to 1-Propyl-3-Methylimidazolium Toluenesulfonate as a reaction medium. This chemical handles high temperatures, lets reactions run more efficiently, and often bumps up yields. For example, in the creation of pharmaceuticals or fine chemicals, researchers find that this liquid shortens reaction times. New drug candidates reach the next stage faster, which matters given the long road from molecule to medicine cabinet. Published studies back this up, noting that ionic liquids have helped cut energy costs by up to 20%, all while delivering more consistent results.
Better Metal Processing
Electroplating and other metal finishing jobs benefit, too. This compound helps form smoother, more uniform layers of metals such as gold or silver. Traditional plating baths usually bring a toxic mix to the table, but this alternative reduces risk and sometimes raises efficiency. While running plating trials, I saw how easy it became to control crystal growth on tricky shapes—something metal shops have fought for decades. Manufacturers need these even coatings, not only for looks but for extending the lifespan of electronics and connectors.
Bringing Out Purity in Extraction
Industries that separate valuable compounds—think biofuels or rare earth elements—see ionic liquids like 1-Propyl-3-Methylimidazolium Toluenesulfonate as a big leap. They pull target chemicals out of complex mixtures more selectively, saving time and cutting back on waste. In biofuel plants that extract sugar from biomass, this approach trims down chemical use and often delivers a cleaner final product. From experience in pilot projects, less chemical waste also cuts disposal costs and lowers regulatory headaches, which can mean smoother approvals and better margins.
Challenges and Solutions
Despite the upside, handling ionic liquids brings real questions: price, shelf stability, and how to reclaim or recycle them. Their up-front cost sits higher than bulk solvents, which can spook small operations. Scaling up recycling programs helps—some plants already recover and reuse upwards of 80% of these liquids by tweaking washing protocols. Chemical engineers keep pushing research into making the base materials from renewable feedstocks, which could lower entry costs in the future. Every industrial shift brings growing pains, but shared learnings from pilot plants, as seen in green chemistry journals and trade conferences, speed up wider adoption.
Looking Ahead
Companies aiming for safer, cleaner factories keep picking ionic liquids like 1-Propyl-3-Methylimidazolium Toluenesulfonate for a reason. Their flexibility bridges gaps in old systems that environmental rules now push to the edge. Watching this trend firsthand, the key lesson stands out: real progress often rides on small, smart changes at the molecular level—ones that protect both people and the world outside the lab.